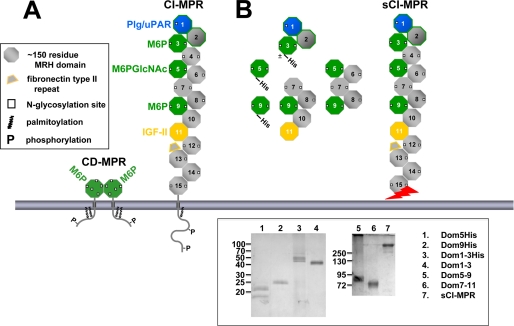
FIGURE 2.
Man-6-P binding sites of the CI-MPR. A, schematic diagram of the full-length CI-MPR and CD-MPR. The MPRs are type I integral membrane glycoproteins, each with an N-terminal signal sequence, an extracytoplasmic region, a single transmembrane region, and a C-terminal cytoplasmic domain. The CI-MPR has a large extracytoplasmic region comprised of 15 contiguous domains, each ∼150 residues in length. The location of the Man-6-P and IGF-II binding sites are indicated. B, the truncated CI-MPR constructs used in this study are shown. Constructs that contain a C-terminal tag of six histidine residues are indicated. The sCI-MPR was purified from fetal bovine serum. The jagged arrow indicates that the C terminus of the sCI-MPR is not defined as this soluble form of the receptor is generated by proteolysis (62, 63). Inset, purified proteins were resolved on 12 (left panel) or 7.5% (right panel) nonreducing SDS-polyacrylamide gels and visualized by silver staining. The two bands observed for the Dom5His construct (lane 1) represent glycosylated (21 kDa) and non-glycosylated (18 kDa) species as determined by endoglycosidase H digestion (data not shown). The slower mobility of the P. pastoris-derived Dom1–3His construct (lane 3, 45–50 kDa) compared with the Sf9-derived Dom1–3 construct (lane 4, 43 kDa) is due to the presence of the six histidine residues and the larger (Man8-Man12) N-glycans produced in P. pastoris (36) compared with the smaller Man3GlcNAc2 N-glycans typically produced in Sf9 insect cells (36).