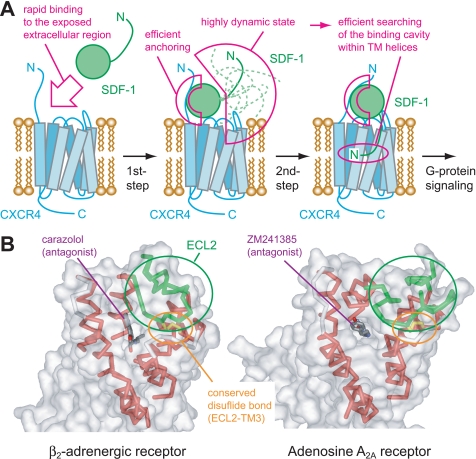
FIGURE 6.
Physiological role of the two-step mechanism for the SDF-1α-CXCR4 interaction. A, schematic diagram of the two-step mechanism for the SDF-1α-CXCR4 interaction. The 1st step interaction between the SDF-1α β-sheet, 50-s loop, and N-loop and the CXCR4 extracellular region facilitates the rapid binding and efficient anchoring of SDF-1α on the extracellular side of CXCR4. The SDF-1α N terminus is highly dynamic even in this state, which is used to search for the binding cavities buried within the TM helices. Consequently, the 2nd step interaction between the SDF-1α N terminus and the CXCR4 TM region is formed, and the SDF-1α N terminus triggers the conformational changes in the CXCR4 TM to induce G-protein signaling. B, crystal structures of two GPCRs, β2-adrenergic receptor (Protein Data Bank code 2RH1), and adenosine A2A receptor (Protein Data Bank code 3EML). Small molecule antagonists (shown as sticks) are buried in the deep cavities within the TM helices (red ribbons). The entrances of these cavities are restricted by the long ECL2s (green ribbons) and the conserved disulfide bonds between ECL2 and TM3 (yellow sticks). Solvent-accessible surfaces of the receptors are also shown (transparent gray surfaces).