Abstract
Zooplankton play an important role in the trophic dynamics of coral reef ecosystems. Detailed vertical and temporal distribution and biomass of zooplankton were evaluated at four heights off the bottom and at six times throughout the diel cycle over a coral reef in the Florida Keys (USA). Zooplankton abundance averaged 4396 ± 1949 SD individuals m−3, but temporal and spatial distributions varied for individual zooplankton taxa by time of day and by height off the bottom. Copepods comprised 93–96% of the abundance in the samples. Taxon-based zooplankton CHN values paired with abundance data were used to estimate biomass. Average daily biomass ranged from 3.1 to 21.4 mg C m−3 and differed by both height off the bottom and by time of day. While copepods were the numerically dominant organisms, their contribution to biomass was only 35% of the total zooplankton biomass. Our findings provide important support for the new emerging paradigm of how zooplankton are distributed over reefs.
INTRODUCTION
Coral reefs have complex assemblages of zooplankton from several sources, including reef resident species, migrating demersal species, open ocean holoplankton advected onto reefs and meroplankton from both open oceans and reef sources (Emery, 1968; Alldredge and King, 1977; Heidelberg et al., 2004). These diverse assemblages provide substantial nutrient input to the reef ecosystem for reef fish, corals and other predators.
The characterization of complete zooplankton assemblages near reef surfaces is challenging due to artifacts of different sampling strategies, topographically complex structures and diel changes in supply of zooplankton. For example, the use of emergence and re-entry traps provide abundances m−2 of only zooplankton that reside within reef surfaces (Emery, 1968; Alldredge and King, 1977; Hammer, 1981; Alldredge and King, 1985). Studies using nets tend to preferentially capture zooplankton that are well above uneven reef surfaces. More recently, communities of zooplankton at scales relevant to benthic zooplanktivores have been described (Heidelberg et al., 2004; Yahel et al., 2005a, b), and a new paradigm is emerging about factors controlling reef-wide community assemblages (Holzman et al., 2005; Motro et al., 2005).
The objectives of this study were to provide a detailed evaluation of depth and time-dependent differences in zooplankton community densities and biomass throughout the diel cycle. We used simultaneous sampling at different heights ranging from centimeters off the bottom to just below the water column surface and at multiple time points (day, dawn/dusk and night) to test whether communities and specific taxa would be evenly distributed by space and time in the water column. A particular emphasis was to focus on the first 2.0 m above the bottom to evaluate differences in zooplankton community assemblages, where interactions with benthic heterotrophs are most likely.
METHOD
Site description and sample collection
The research site was a shallow coral reef within the Florida Keys National Marine Sanctuary that is associated with the NOAA National Underwater Research Center Aquarius laboratory on Conch Reef (24°57.0′ N, 80°27.3′ W). Conch Reef is a Holocene reef located approximately 5–8 km offshore along the Pleistocene margin of south Florida (Shinn et al., 1989; Aronson et al., 1994). In summer months, the water column is typically well stratified with a warm, isothermal surface layer to approximately 50–80 m depth (e.g. Leichter et al., 1998; Leichter et al., 2003). Tidal bores are a constant feature of the physical environment at Conch Reef, occurring up to 10–20% of the time during summer months (Leichter et al., 1996, 1998, 2003). To date, zooplankton community assemblages have not been intensively studied at this otherwise well-studied site.
Sampling was carried out on a section of spur and groove reef 15 m deep adjacent to a deeper, sand channel on multiple days between 10 and 17 July 2000. Two identical multi-intake plankton pumps modified from a previous study (Heidelberg et al., 2004) (Fig. 1) were used to sample simultaneously at four heights off the bottom: substratum, 0.5, 1.0 and 2.0 m above the bottom (mab) and at the six predetermined times of 00.00, 03.00, 06.00, 12.00, 18.00 and 21.00 h. Sunrise on 11 July was at 06.40 h and sunset was at 20.15 h, making 06.00 h samples 40 min prior to sunrise and 21.00 h samples 45 min after sunset. Therefore, our design included two day samples (12:00 h and 18:00 h), two samples from dusk/dawn (06.00 and 21.00 h), and two night samples (00.00 and 03.00 h) for each 24 h period. Simultaneous near surface samples were collected for four of the seven sampling dates (13–17 July), at 2.0 m depth below the surface using a separate single-entry zooplankton pump (Rule 1200) with a collection hose extending from the habitat to near surface.
Fig. 1.
One of two zooplankton pump arrays used for simultaneous sampling from multiple heights. Surface pump is not shown. Inset shows a close up of omni-directional and lateral intake head. Near substrate intake head was inverted to allow placement within a few cm of substrate. Samples from the 1.0 mab were not included in this study (mab, meters above bottom).
Pump arrays were constructed of four intake tubes made of 7.6 cm diameter PVC pipe powered by a 24 volt DC bilge pump (Rule 2500) at the base. Pumps were calibrated both in a swimming pool and during deployment. Total pump rates for each pump were evaluated by measuring time required to fill a known volume with excurrent flows. An acoustic-Doppler velocimeter (SonTek/YSI 10-MHz ADV) was used to measure flow speeds at the center of each tube of the four-intake pump array. Each pump intake was configured by adjusting relative position and length of PVC between different intake heads and outlet such that pump flow rates at each intake head had almost identical velocities.
Flow into the intake heads was omni-directional and lateral (intake direction irrespective of ambient flow direction), and flow at the intake was more rapid than swimming speeds of most zooplankters (>30 cm s−1) as previously tested (Sebens et al., 1996). The substrate intake head was inverted to be positioned within a few centimeters of benthic surfaces. Zooplankton samples were collected in 40 µm Nitex mesh bags just downstream of the intake heads and prior to going through the pump rotor, to minimize damage. Pump operation was controlled through diver radio-communications with technicians inside the Aquarius habitat. Nets were closed inside vertical inlet tubes by divers immediately after the pump was turned off to prevent sample loss, collected and samples preserved in 5% formalin in seawater.
Determination of zooplankton abundance
A total of 210 samples were collected for this study (Table I). A few samples were not analyzed due to evidence of inadequate preservation. Entire samples or subsamples (5 ml each) were taken from a well-mixed sample using a Stempel pipette (Omori and Ikeda, 1984) and enumerated using a Ward zooplankton counting wheel (Wildlife Supply Co.) and Leica dissecting microscope. Samples were counted until at least 200 individuals were identified for each sample. Copepods were identified at least to genus following Owre and Foyo (Owre and Foyo, 1967) and Huys and Boxshall (Huys and Boxshall, 1991) with assistance from A. Willey (Univ. Maryland, Horn Point Laboratory.). Other organisms were identified to order or other higher taxon. The first 10 of each genus/taxon in each sample were measured using an optical micrometer. Abundances of individual taxa were standardized to numbers m−3 based on the volume of water pumped for each sample. The effects of depth and time of day on abundance were tested for total zooplankton and for individual taxa when abundant enough for adequate replication.
Table I:
Sampling effort
| Pump array 1 | Pump array 2 | Surface pump | Total | |
|---|---|---|---|---|
| Sample volume (range) | 0.63 ± 0.06 SD m−3 (0.57–0.78 m−3) | 0.62 ± 0.08 SD m−3 (0.54–0.99 m−3) | 0.34 ± 0.08 SD m−3 (0.18–0.59 m−3) | |
| Near-substrate | 26 | 35 | 61 | |
| 0.5 mab | 27 | 36 | 63 | |
| 2.0 mab | 26 | 37 | 63 | |
| Near Surface | 24 | 211 |
We collected 211 samples over the course of this study using 2 replicate pump arrays (Fig. 1) and a single surface pump (see text). Average pump sample volumes shown. There were only 4 days with complete replication from all depths and times. When replicate samples were present for the same height and time, they were averaged prior to undergoing a two-way repeated measures ANOVA analysis. All pumps were run for ∼20 min for each sample.
Measurements of zooplankton biomass
Zooplankton biomass values were obtained for each zooplankton group in the following manner. Live zooplankton were collected on the reef by using a light to attract zooplankton into a net. The net was immediately transported to the open entrance (wet porch) of the habitat, where zooplankton were gently filtered onto a 64 µm Nitex screen. The screen was chilled for 1 min to decrease zooplankton activity. Live zooplankton were measured using a dissecting microscope fitted with an ocular micrometer. They were then sorted onto replicate, ashed and pre-weighed Whatman GF/C filters in dishes divided into type and size range (e.g. Acartia spp. copepods were sorted into three replicates of three size range categories). Zooplankton were then immediately transferred to the surface and dried at 60°C. Carbon and nitrogen contents of each zooplankton type were measured by the Dumas combustion method on a CHN analyzer. The measured value of carbon for each filter was divided by the number of organisms on the filter to obtain estimates for each organism in each size range grouping (Table II). Biomass per sample was calculated by multiplying the measured value by the abundance of each type of zooplankton at a specific size and summing the values for each sample. There were some organism types that we did not collect in our CHN sampling. For these organisms, we estimated biomass in the following way. Size-based literature values (Glynn, 1973; Uye, 1982; Roman et al., 1990) were combined with our field samples values. Regression equations were generated for zooplankton carbon content in µg C by taxon and size. Copepod carbon content was estimated using the regression equation: LN Copepod biomass = 1.82 * LN(S) + 1.28 (r2 = 0.893; df = 16; F = 125: sig. = 1.12 × 10−8), where LN is the natural logarithm and S is body length in mm. Carbon content for other zooplankton taxa were estimated using the equation: LN Other Biomass = 1.46 * LN(S) + 1.03 (r2 = 0.733; df = 16; F = 80.7; sig. = 3.47 × 10−7). Size-based, log-transformed values estimated from regression equations were back transformed prior to multiplying by abundances.
Table II:
Size and taxon based carbon and nitrogen biomass values measured as part of this study
| Zooplankton ID | Size range (µm) | No. of ZP | Average size (µm) | C ind. (µg) | N ind. (µg) | C:N ratio |
|---|---|---|---|---|---|---|
| Amphipod, gammarid | 1500–2000 | 10 | 1788.2 | 24.7 | 6.0 | 4.15 |
| Amphipod, gammarid | 3000–3500 | 6 | 3300.0 | 136.6 | 31.6 | 4.32 |
| Amphipod, gammarid | 3500–4000 | 8 | 3743.8 | 141.5 | 33.7 | 4.20 |
| Amphipod, gammarid | 4000–4500 | 8 | 4305.0 | 274.5 | 69.3 | 3.96 |
| Amphiipod, hyperid | 1500–2000 | 8 | 1794.6 | 36.1 | 7.9 | 4.55 |
| Amphiipod, hyperid | 2500–3000 | 8 | 2687.6 | 74.4 | 16.8 | 4.44 |
| Chaetognath | 5500–6500 | 4 | 5894.3 | 37.9 | 10.4 | 3.64 |
| Chaetognath | 7000–7500 | 3 | 7269.0 | 62.8 | 17.8 | 3.54 |
| Chaetognath | 8000–8700 | 5 | 8455.4 | 90.3 | 25.5 | 3.54 |
| Chaetognath | 9000–12 000 | 3 | 11 384.3 | 119.9 | 33.1 | 3.62 |
| Copepod, calanoid (Acartia) | 900–1200 | 10 | 1031.9 | 2.9 | 0.7 | 4.09 |
| Copepod, calanoid (Acartia) | 900–1200 | 11 | 1066.2 | 2.5 | 0.6 | 3.88 |
| Copepod, calanoid, misc. | 500–999 | 10 | 788.1 | 1.8 | 0.4 | 4.08 |
| Copepod, calanoid (mostly Acartia) | 1000–1500 | 10 | 1141.6 | 6.3 | 1.7 | 3.76 |
| Copepod, calanoid, misc. | 2000–2500 | 8 | 2193.1 | 51.7 | 14.7 | 3.52 |
| Copepod, calanoid (Labidocera) | 2300–2700 | 8 | 2620.5 | 76.5 | 22.1 | 3.46 |
| Copepod, cyclopoid, misc. | 250–500 | 5 | 438.2 | 0.3 | 0.1 | 3.86 |
| Copepod, cyclopoid, misc. | 750–1000 | 4 | 908.5 | 1.2 | 0.2 | 5.06 |
| Copepod, harpacticoid, misc. | 400–1000 | 6 | 728.5 | 2.4 | 0.4 | 5.55 |
| Copepod, Monstrilla | 2800–3900 | 8 | 3210.6 | 55.7 | 11.6 | 4.80 |
| Cumacean | 2000–2500 | 10 | 2150.0 | 13.3 | 2.4 | 5.44 |
| Cumacean | 6000–6500 | 3 | 6166.7 | 196.9 | 40.2 | 4.90 |
| Decapod, crab zoea | 500–1000 | 4 | 936.2 | 18.0 | 3.6 | 4.94 |
| Decapod, crab zoea | 1000–1500 | 10 | 1252.7 | 19.6 | 4.1 | 4.74 |
| Decapod, crab zoea | 2000–2500 | 8 | 2141.6 | 15.1 | 3.3 | 4.52 |
| Decapod larvae, misc. | 3000–3500 | 5 | 3354.4 | 77.3 | 19.7 | 3.92 |
| Decapod larvae, misc. | 5000–5500 | 5 | 5292.2 | 153.9 | 42.0 | 3.67 |
| Decapod larvae, misc. | 7000–7500 | 2 | 7275.0 | 273.6 | 67.6 | 4.05 |
| Isopods | 1100–1600 | 10 | 1441.0 | 14.5 | 2.3 | 6.41 |
| Isopods | 2100–2600 | 10 | 2447.7 | 80.3 | 17.3 | 4.63 |
| Isopods | 2600–3100 | 8 | 2865.4 | 94.8 | 20.7 | 4.58 |
| Isopods | 3400–4000 | 5 | 3761.6 | 188.8 | 38.8 | 4.87 |
| Mysid shrimp | 4000–4500 | 8 | 4183.6 | 87.2 | 24.2 | 3.60 |
| Mysid shrimp | 5000–5500 | 5 | 5175.2 | 211.8 | 58.6 | 3.61 |
| Mysid shrimp | 6000–6500 | 3 | 6165.3 | 177.4 | 48.7 | 3.65 |
| Mysid shrimp | 7000–8000 | 3 | 7499.7 | 362.0 | 100.7 | 3.59 |
| Ostracoda | 600–1100 | 4 | 850.5 | 4.6 | 0.7 | 6.37 |
| Polychaetes | 2000–3000 | 5 | 2900.0 | 26.4 | 6.1 | 4.31 |
| Polychaetes | 3500–4500 | 5 | 3974.4 | 58.6 | 13.4 | 4.38 |
| Polychaetes | 6000–7000 | 3 | 6296.0 | 100.2 | 26.6 | 3.8 |
| Polychaetes | 8000–8500 | 1 | 8350.0 | 123.4 | 28.7 | 4.3 |
| Polychaetes | 30 000 | 1 | 30 000.0 | 1707.0 | 335.5 | 5.1 |
| Stomatopod larvae | 4000–4500 | 2 | 4625.0 | 63.1 | 15.5 | 4.1 |
| Stomatopod larvae | 5000–6000 | 5 | 5253.6 | 142.7 | 37.0 | 3.9 |
| Stomatopod larvae | 7000–8000 | 2 | 7691.5 | 638.0 | 149.9 | 4.3 |
| Stomatopod larvae | 14 000 | 1 | 14 000.0 | 924.9 | 215.9 | 4.3 |
Statistical Analyses
Zooplankton data were statistically analyzed using Statistica (ver. 8, StaSoft) software. Homogeneity of variance was assessed using Hartley, F-max, Cochran C and Bartlett Chi-square analysis at the appropriate level of degrees of freedom. A repeated measures two-way ANOVA analysis was undertaken on four complete daily cycles (4 days × 4 heights × 6 times) of means or transformed mean densities for all common groups or subgroups to evaluate effects of depth and time on community structure. Data were also evaluated by the Mauchley sphericity test to validate repeated measures ANOVAs (Anderson, 1958). If there was a significant violation of sphericity, an adjusted (Huynh–Feldt) univariate procedure for repeated measures was used. If the ANOVA produced a significant result, a posteriori pair-wise comparisons were undertaken with a Fisher LSD analysis. A summary of needed transformations and statistical outcomes for common zooplankton groups is presented in Table III. Most common groups were also graphically represented to show detailed results of pair-wise comparisons.
Table III:
Summary of statistical approaches and results for repeated measures two-way ANOVA analysis on the effects of depth, time and the interaction of depth and time on mean abundance of common zooplankton taxa
| Plankter copepods | Trans. | Depth (df = 3) |
Time (df = 5) |
Depth × Time (df = 15) |
Spher. | |||
|---|---|---|---|---|---|---|---|---|
| P-value | F | P-value | F | P-value | F | |||
| Calanoids | ||||||||
| Acartia | Log | 0.004 | 7.435 | 0.000 | 10.239 | 1.126 | 0.354 | sig |
| Calanopia | Log | 0.853 | 0.263 | 0.006 | 3.569 | 0.285 | 1.22 | ns |
| Calanus | Log | 0.000 | 50.445 | 0.044 | 2.489 | 0.457 | 1.01 | ns |
| Calocalanus | Log | 0.538 | 0.744 | 0.826 | 0.436 | 0.642 | 0.82 | ns |
| Paracalanus | Log | 0.451 | 0.903 | 0.003 | 3.990 | 0.011 | 2.46 | ns |
| Temora | LN + 1 | 0.000 | 17.400 | 0.255 | 1.353 | 0.888 | 0.567 | ns |
| Calanoid unid. | Log | 0.002 | 5.561 | 0.041 | 2.421 | 0.585 | 0.88 | ns |
| Harpacticoids | ||||||||
| Tegastidae family | Log | 0.000 | 13.912 | 0.001 | 4.921 | 0.000 | 9.88 | ns |
| Macrosetella | Log | 0.156 | 1.858 | 0.002 | 5.259 | 0.448 | 1.02 | sig |
| Microsetella | Sqrt | 0.000 | 15.525 | 0.629 | 0.676 | 0.303 | 1.192 | ns |
| Laophontidae family | Log | 0.000 | 18.27 | 0.079 | 2.053 | 0.598 | 0.87 | ns |
| Cyclopoids | ||||||||
| Oithona | Sqrt | 0.028 | 4.310 | 0.472 | 0.924 | 0.719 | 0756 | ns |
| cyclopoid unid | Log | 0.644 | 0.560 | 0.048 | 2.337 | 0.009 | 2.432 | sig |
| Poecilostomatoids | ||||||||
| Copilia | Log | 0.000 | 24.23 | 0.003 | 3.906 | 0.098 | 1.59 | ns |
| Corycaeus | Sqrt | 0.000 | 14.884 | 0.132 | 1.773 | 0.890 | 0.565 | ns |
| Oncaea | Sqrt | 0.189 | 1.635 | 0.025 | 3.264 | 0.486 | 0.98 | ns |
| Copepodites | Log | 0.006 | 5.343 | 0.000 | 6.220 | 0.000 | 3.75 | ns |
| Copepod nauplii | Sqrt | 0.000 | 15.343 | 0.626 | 0.699 | 0.529 | 0.938 | ns |
| Exoskeletons | Sqrt | 0.127 | 3.098 | 0.098 | 2.773 | |||
| Non-copepod | ||||||||
| Amphipods | Sqrt | 0.359 | 1.176 | 0.029 | 2.698 | 0.569 | 0.898 | sig |
| Chaetognaths | Sqrt | 0.008 | 6.418 | 0.384 | 1.074 | 0.0690 | 1.729 | ns |
| Decapods, crab zoea | LN + 1 | 0.001 | 14.39 | 0.002 | 4.130 | 0.040 | 1.912 | ns |
| Fish eggs | Sqrt | 0.000 | 171.97 | 0.000 | 5.447 | 0.064 | 1.755 | sig |
| Isopods | Sqrt | 0.005 | 7.215 | 0.296 | 1.252 | 0.910 | 0.535 | ns |
| Larvaceans | LN + 1 | 0.000 | 23.717 | 0.069 | 2.177 | 0.653 | 0.819 | ns |
| Ostracods | Log | 0.148 | 2.143 | 0.000 | 5.159 | 0.262 | 1.251 | ns |
| Polychaetes | Log | 0.084 | 2.31 | 0.11 | 1.85 | 0.827 | 0.65 | ns |
| Total zooplankton | Sqrt | 0.001 | 10.46 | 0.443 | 0.970 | 0.965 | 0.501 | ns |
| Total copepods | Sqrt | 0.009 | 6.109 | 0.070 | 2.167 | 0.638 | 0.834 | ns |
| Total non-copepods | Sqrt | 0.003 | 4.97 | 0.001 | 4.47 | 0.716 | 0.79 | ns |
| Total decapods | Log | 0.003 | 4.95 | 0.000 | 18.96 | 0.716 | 0.76 | ns |
Only groups that passed ANOVA assumptions are shown. For significant ANOVA tests, a Mauchley–Sphericity test was performed (see text).
RESULTS
Abundance and distribution of zooplankton communities
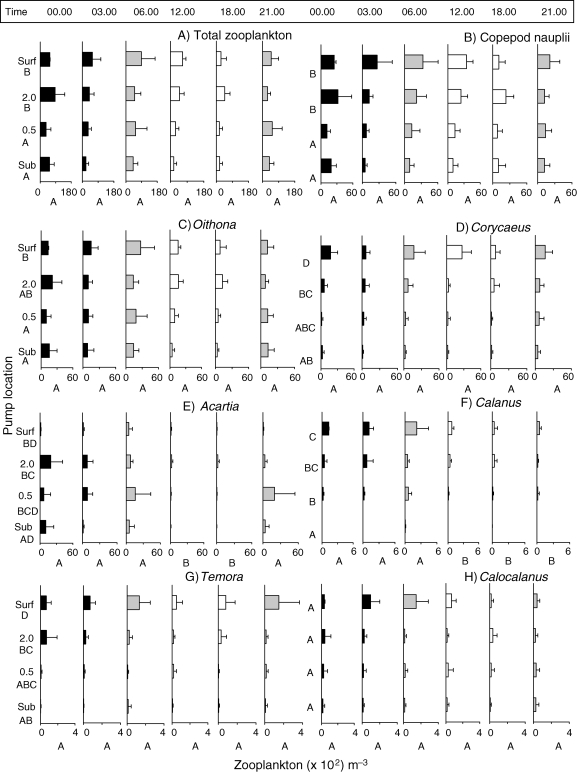
Total mean zooplankton abundance, from all times and depths, was 4396 ± 1949 SD individuals m−3. There was no significant difference in total zooplankton abundance by time of day, but densities were significantly lower at both the substrate and at 0.5 mab than at either 2.0 mab or at the surface (Fig. 2A; P = 0.001; F = 10.46). Mean zooplankton abundance was also compared by periods in the daily cycle (means of two samples each for day, dawn/dusk and night samples) (Table IV). Using these groupings, mean daytime zooplankton were less abundant than dawn/dusk or night time abundances.
Fig. 2.
Abundance (mean × 102 ± 1 SD) of total zooplankton and of the most-abundant copepod genera. Each figure is made up of six panels ordered by time of day (00.00 to 21.00 h) and by height off the substrate: near substrate, 0.5 and 2.0 m above the bottom (mab) and surface. Letter designations indicate a posteriori significant differences according to a two-way repeated measures Analysis of variance at the P < 0.05 significance. Abundance bars are colored to reflect light levels (white, day; grey, within 45 min of dawn/dusk; black, night). Note differences in x-axis scale for each panel.
Table IV:
Zooplankton abundance (mean ± 1 SD m−3) and biomass (mg C m−3) over three divisions of time: day (12.00 and 18.00 h), dawn/dusk (06.00 and 21.00 h) and night (00.00 and 03.00 h) at the four depths: near substrate, 0.5 and 2.0 m above bottom (mab) and surface
| Near substrate |
0.5 mab |
2.0 mab |
Near surface |
|||||
|---|---|---|---|---|---|---|---|---|
| m−3 | Biomass | m−3 | Biomass | m−3 | Biomass | m−3 | Biomass | |
| Total | 3139.9 ± 571.0 | 8.66 | 3813.8 ± 1099.5 | 12.99 | 4990.8 ± 371.0 | 14.51 | 5588.9 ± 994.8 | 19.21 |
| Day | 1804.7 ± 86.2 | 3.5 ± 0.6 | 2229.6 ± 706.5 | 4.2 ± 1.0 | 4805.0 ± 110.2 | 7.5 ± 0.0 | 4637.1 ± 2761.8 | 10.76 ± 13.9 |
| Dawn/Dusk | 3966.3 ± 205.0 | 11.9 ± 0.3 | 5488.6 ± 63.8 | 19.2 ± 1.2 | 3921.9 ± 1576.0 | 13.7 ± 3.9 | 6848.2 ± 2868.6 | 23.45 ± 4.1 |
| Night | 3717.9 ± 2359.9 | 11.8 ± 6.9 | 3369.6 ± 68.3 | 14.2 ± 4.2 | 6333.3 ± 3281.8 | 23.4 ± 16.9 | 5631.9 ± 131.6 | 22.69 ± 0.7 |
Copepod communities
Copepods dominated all samples, making up 93–96% of organisms in each sample (Table V). Copepod communities comprised many genera, which are detailed in Table VI. Naupliar stages were the most abundant taxa at all depths averaging up to 43% of abundance. They were found in significantly higher numbers at 2.0 mab and at the surface then at 0.5 mab and the substrate (P ≤ 0.001), but did not differ by time of day (Fig. 2B). Because of their high abundances, naupliar patterns statistically affected patterns seen in total zooplankton assemblage. When nauplii were removed from the analysis, total zooplankton assemblages were significantly lower during the day (P = 0.023). The next most abundant group of organisms was the later-stage copepods, comprising 61–69% of the total (Tables V and VI).
Table V:
Zooplankton abundance m−3 (mean ± 1 SD), percent composition and biomass (mg C m−3) for major zooplankton groups at the four depths: near-substrate, 0.5 and 2.0 m off the bottom (mab) and surface
| Zooplankton taxon | Near substrate |
0.5 mab |
2.0 mab |
Near surface |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abund. | % | Biom. | % | Abund. | % | Biom. | % | Abund. | % | Biom. | % | Abund. | % | Biom. | % | |
| Annelids | ||||||||||||||||
| Polychaetes | 23.8 ± 2.4 | 0.8 | 1.835 | 21.2 | 28.7 ± 4.0 | 0.8 | 2.214 | 17.0 | 32.2 ± 5.3 | 0.6 | 2.485 | 17.1 | 65.2 ± 11.2 | 1.2 | 5.028 | 26.2 |
| Arthropods | ||||||||||||||||
| Amphipods | 2.2 ± 0.4 | 0.1 | 0.256 | 3.0 | 3.0 ± 0.7 | 0.1 | 0.433 | 3.3 | 2.8 ± 0.8 | 0.1 | 0.394 | 2.7 | 0.6 ± 0.3 | <0.1 | 0.058 | 0.3 |
| Cirripedia nauplii | 4.2 ± 2.5 | 0.1 | 0.001 | <0.1 | 1.0 ± 0.9 | <0.1 | 0.000 | <0.1 | 1.2 ± 0.3 | <0.1 | 0.000 | 0.0 | 0.9 ± 0.7 | <0.1 | 0.000 | <0.1 |
| Cirripedia other | 3.5 ± 0.5 | 0.1 | 0.008 | 0.1 | 3.9 ± 3.5 | 0.1 | 0.009 | 0.1 | 1.7 ± 1.4 | <0.1 | 0.004 | 0.0 | NP | |||
| Copepods | 2980.1 ± 538.7 | 95.1 | 3.082 | 35.6 | 3625.3 ± 1062.1 | 95.1 | 4.983 | 38.3 | 4788.9 ± 366.9 | 96.0 | 5.276 | 36.4 | 5205.9 ± 799.0 | 93.3 | 5.749 | 29.9 |
| Cumaceans | 1.5 ± 1.8 | <0.1 | 0.160 | 1.8 | 0.5 ± 0.2 | <0.1 | 0.049 | 0.4 | 0.8 ± 0.1 | <0.1 | 0.081 | 0.6 | 0.1 ± 0.0 | 0.0 | 0.015 | 0.1 |
| Decapods, zoea | 0.9 ± 0.2 | <0.1 | 0.017 | 0.2 | 10.4 ± 2.1 | 0.3 | 0.186 | 1.4 | 7.6 ± 3.0 | 0.2 | 0.136 | 0.9 | 5.5 ± 3.1 | 0.1 | 0.099 | 0.5 |
| Decapoda, other | 3.3 ± 0.3 | 0.1 | 0.555 | 6.4 | 10.1 ± 1.0 | 0.3 | 1.694 | 13.0 | 17.7 ± 7.1 | 0.4 | 2.976 | 20.5 | 31.7 ± 21.9 | 0.6 | 5.335 | 27.8 |
| Isopods | 14.5 ± 2.8 | 0.5 | 1.371 | 15.8 | 11.9 ± 8.1 | 0.3 | 1.122 | 8.6 | 11.7 ± 6.4 | 0.2 | 1.111 | 7.7 | 1.1 ± 0.2 | <0.1 | 0.104 | 0.5 |
| Arachnida (mites) | 0.9 ± 0.0 | <0.1 | 0.000 | <0.1 | 0.4 ± 0.5 | <0.1 | 0.000 | 0.0 | 1.2 ± 1.3 | <0.1 | 0.000 | <0.1 | 0.1 ± 0.0 | <0.1 | 0.000 | <0.1 |
| Mysids | 1.3 ± 0.8 | <0.1 | 0.262 | 3.0 | 3.3 | 0.1 | 0.701 | 5.4 | 1.0 ± 0.0 | <0.1 | 0.212 | 1.5 | NP | |||
| Ostracods | 29.5 ± 10.0 | 0.9 | 0.135 | 1.6 | 20.8 | 0.5 | 0.095 | 0.7 | 6.0 ± 3.0 | 0.1 | 0.028 | 0.2 | 17.2 ± 7.3 | 0.3 | 0.079 | 0.4 |
| Pycnogonids | NP | 0.2 ± 0.3 | <0.1 | 0.001 | <0.1 | 0.1 ± 0.0 | <0.1 | 0.000 | <0.1 | NP | ||||||
| Stomatopods | 0.4 ± 0.2 | <0.1 | 0.365 | 4.2 | 0.7 ± 0.5 | <0.1 | 0.630 | 4.8 | 0.7 ± 0.8 | <0.1 | 0.627 | 4.3 | 0.1 ± 0.1 | <0.1 | 0.134 | 0.7 |
| Tanaids | 0.2 ± 0.2 | <0.1 | 0.027 | 0.3 | <0.1 | <0.1 | 0.005 | <0.1 | NP | NP | ||||||
| Chaetognaths | 6.7 ± 0.2 | 0.2 | 0.523 | 6.0 | 10.1 ± 6.3 | 0.3 | 0.785 | 6.0 | 13.1 ± 1.5 | 0.3 | 1.018 | 7.0 | 24.1 ± 8.3 | 0.4 | 1.875 | 9.8 |
| Chordates | ||||||||||||||||
| Fish eggs | 40.4 ± 29.4 | 1.3 | 0.011 | 0.1 | 67.4 ± 22.5 | 1.8 | 0.019 | 0.1 | 53.5 ± 1.6 | 1.1 | 0.015 | 0.1 | 55.9 ± 28.2 | 1.0 | 0.016 | 0.1 |
| Fish larvae | NP | 0.4 ± 0.4 | <0.1 | 0.040 | 0.3 | 0.1 ± 0.2 | <0.1 | 0.014 | 0.1 | 1.9 ± 0.7 | <0.1 | 0.196 | 1.0 | |||
| Salps | 1.7 ± 2.4 | 0.1 | 0.007 | 0.1 | <0.1 | <0.1 | 0.000 | <0.1 | NP | NP | ||||||
| Larvaceans | 10.1 ± 4.1 | 0.3 | 0.033 | 0.4 | 8.3 ± 3.3 | 0.2 | 0.027 | 0.2 | 31.1 ± 14.6 | 0.6 | 0.100 | 0.7 | 151.5 ± 71.7 | 2.7 | 0.488 | 2.5 |
| Cnidaria | 0.1 ± 0.2 | <0.1 | 0.000 | <0.1 | 3.3 ± 0.3 | 0.1 | 0.000 | <0.1 | 0.8 ± 0.5 | <0.1 | 0.001 | <0.1 | NP | |||
| Echinoderms | 2.8 ± 0.4 | 0.1 | 0.001 | <0.1 | 2.2 ± 1.7 | 0.1 | 0.000 | <0.1 | 4.5 ± 0.8 | 0.1 | 0.001 | 0.0 | 13.9 ± 6.9 | 0.2 | 0.003 | 0.0 |
| Molluscs | ||||||||||||||||
| Gastropods | 4.3 ± 0.3 | 0.1 | 0.013 | 0.1 | 0.1 ± 0.1 | <0.1 | 0.000 | <0.1 | 9.8 ± 5.1 | 0.2 | 0.030 | 0.2 | 12.1 ± 5.2 | 0.2 | 0.036 | 0.2 |
| Veligers | NP | NP | 0.4 ± 0.5 | <0.1 | 0.001 | <0.1 | NP | |||||||||
NP, not present.
Table VI:
Zooplankton abundance m−3 (mean ± 1 SD), percent composition and biomass (mg C m−3) for copepods at the four depths: near-substrate, 0.5 and 2.0 m off the bottom (mab) and surface
| Zooplankton taxon | Near substrate |
0.5 mab |
2.0 mab |
Near surface |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abund. | % | Biom. | % | Abund. | % | Biom | % | Abund. | % | Biom | % | Abund. | % | Biom. | % | |
| Calanoids | ||||||||||||||||
| Acartia | 334.6 ± 65.8 | 11.2 | 0.904 | 29.4 | 893.5 ± 236.4 | 24.6 | 2.414 | 48.5 | 718.4 ± 90.2 | 15.0 | 1.941 | 36.8 | 157.6 ± 62.0 | 3.0 | 0.426 | 6.4 |
| Calanopia | 3.7 ± 0.0 | 0.1 | 0.024 | 0.8 | 8.5 ± 5.2 | 0.2 | 0.053 | 1.1 | 6.4 ± 2.8 | 0.1 | 0.040 | 0.8 | 4.9 ± 3.1 | 0.1 | 0.031 | 0.5 |
| Calanus | 2.0 ± 2.8 | 0.1 | 0.008 | 0.3 | 83.8 ± 41.1 | 2.3 | 0.340 | 6.8 | 141.7 ± 40.6 | 3.0 | 0.574 | 10.9 | 327.4 ± 62.7 | 6.3 | 1.327 | 19.8 |
| Calocalanus | 16.5 ± 10.4 | 0.6 | 0.030 | 1.0 | 24.2 ± 12.6 | 0.7 | 0.044 | 0.9 | 25.4 ± 4.3 | 0.5 | 0.046 | 0.9 | 68.0 ± 20.5 | 1.3 | 0.124 | 1.8 |
| Candacia | 0.1 ± 0.1 | <0.1 | 0.009 | 0.3 | 3.5 ± 4.6 | 0.1 | 0.234 | 4.7 | 0.7 ± 0.5 | <0.1 | 0.043 | 0.8 | 2.0 ± 1.9 | <0.1 | 0.133 | 2.0 |
| Eucalanus | 0.1 ± 0.1 | <0.1 | 0.000 | <0.1 | 0.4 ± 0.6 | <0.1 | 0.002 | <0.1 | 1.8 ± 1.4 | <0.1 | 0.007 | 0.1 | 0.4 ± 0.3 | <0.1 | 0.002 | <0.1 |
| Labidocera | 0.2 ± 0.2 | <0.1 | 0.017 | 0.6 | 0.6 ± 0.3 | <0.1 | 0.045 | 0.9 | 0.1 ± 0.0 | <0.1 | 0.010 | 0.2 | 1.7 ± 0.5 | <0.1 | 0.133 | 2.0 |
| Paracalanus | 34.6 ± 11.7 | 1.2 | 0.140 | 4.5 | 5.9 ± 4.2 | 0.2 | 0.024 | 0.5 | 25.0 ± 13.8 | 0.5 | 0.101 | 1.9 | 23.1 ± 9.9 | 0.4 | 0.094 | 1.4 |
| Temora | 6.4 ± 4.5 | 0.2 | 0.040 | 1.3 | 10.8 ± 4.6 | 0.3 | 0.068 | 1.4 | 26.8 ± 8.2 | 0.6 | 0.169 | 3.2 | 93.4 ± 51.0 | 1.8 | 0.588 | 8.8 |
| Calanoid unid. | 39.1 ± 4.2 | 1.3 | 0.159 | 5.1 | 84.5 ± 34.8 | 2.3 | 0.343 | 6.9 | 164.0 ± 18.5 | 3.4 | 0.665 | 12.6 | 257.2 ± 93.2 | 4.9 | 1.043 | 15.6 |
| Harpacticoids | ||||||||||||||||
| Tegastidae | 39.3 ± 11.0 | 1.3 | 0.096 | 3.1 | 15.8 ± 1.0 | 0.4 | 0.039 | 0.8 | 3.8 ± 0.8 | 0.1 | 0.009 | 0.2 | NP | |||
| Laophontidae | 213.5 ± 29.8 | 7.2 | 0.522 | 16.9 | 79.7 ± 23.3 | 2.2 | 0.195 | 3.9 | 60.2 ± 24.4 | 1.3 | 0.147 | 2.8 | 20.3 ± 6.6 | 0.4 | 0.050 | 0.7 |
| Macrosetella | 0.7 ± 0.4 | <0.1 | 0.002 | 0.1 | 1.9 ± 0.2 | 0.1 | 0.005 | 0.1 | 3.6 ± 3.2 | 0.1 | 0.009 | 0.2 | 10.7 ± 3.2 | 0.2 | 0.026 | 0.4 |
| Microsetella | 4.6 ± 3.9 | 0.2 | 0.011 | 0.4 | 5.8 ± 0.1 | 0.2 | 0.014 | 0.3 | 8.1 ± 1.9 | 0.2 | 0.020 | 0.4 | 25.5 ± 11.8 | 0.5 | 0.062 | 0.9 |
| Unidentified | 3.3 ± 0.6 | 0.1 | 0.008 | 0.3 | 2.4 ± 2.6 | 0.1 | 0.006 | 0.1 | 1.7 ± 1.1 | <0.1 | 0.004 | 0.1 | 8.5 ± 2.1 | 0.2 | 0.021 | 0.3 |
| Cyclopoids | ||||||||||||||||
| Oithona | 962.6 ± 212.0 | 32.3 | 0.718 | 23.3 | 1117.7 ± 429.4 | 30.8 | 0.834 | 16.7 | 1359 ± 157.6 | 28.4 | 1.014 | 19.2 | 1510 ± 301.8 | 29.0 | 1.126 | 16.8 |
| Cyclopoid unid | 23.6 ± 2.0 | 0.8 | 0.018 | 0.6 | 15.3 ± 2.4 | 0.4 | 0.012 | 0.2 | 15.1 ± 2.7 | 0.3 | 0.011 | 0.2 | 13.6 ± 5.7 | 0.3 | 0.010 | 0.2 |
| Poecilostomatoids | ||||||||||||||||
| Copilia | 12.8 ± 0.2 | 0.4 | 0.031 | 1.0 | 3.6 ± 1.5 | 0.1 | 0.009 | 0.2 | 2.6 ± 00.7 | 0.1 | 0.006 | 0.1 | NP | |||
| Corycaeus | 24.8 ± 10.8 | 0.8 | 0.007 | 0.2 | 31.3 ± 20.1 | 0.9 | 0.009 | 0.2 | 57.9 ± 11.8 | 1.2 | 0.016 | 0.3 | 149.7 ± 34.0 | 2.9 | 0.042 | 0.6 |
| Oncaea | 12.4 ± 3.0 | 0.4 | 0.009 | 0.3 | 3.2 ± 1.9 | 0.1 | 0.002 | <0.1 | 7.6 ± 0.9 | 0.2 | 0.006 | 0.1 | 9.3 ± 7.9 | 0.2 | 0.007 | 0.1 |
| Sapphirella | 1.4 ± 0.0 | <0.1 | 0.001 | <0.1 | 3.8 ± 1.6 | 0.1 | 0.003 | 0.1 | 4.3 ± 5.6 | 0.1 | 1.9 ± 0.43 | <0.1 | 0.002 | <0.1 | ||
| Sapphirina | 1.5 ± 0.1 | 0.1 | 0.000 | <0.1 | 3.6 ± 1.1 | 0.1 | 0.001 | <0.1 | NP | 0.7 ± 0.2 | <0.1 | 0.000 | <0.1 | |||
| Monstrilloids | 0.2 ± 0.2 | <0.1 | 0.009 | 0.3 | 0.3 ± 0.4 | <0.1 | 0.016 | 0.3 | NP | 0.1 ± 0.2 | 0.008 | 0.1 | ||||
| Copepodites | 151.6 ± 55.8 | 5.1 | 0.118 | 3.8 | 81.7 ± 39.3 | 2.3 | 0.064 | 1.3 | 72.0 ± 9.3 | 1.5 | 0.056 | 1.1 | 58.7 ± 5.1 | 1.1 | 0.046 | 0.7 |
| Nauplii | 1091 ± 205.5 | 36.6 | 0.200 | 6.5 | 1143.7 ± 211.5 | 31.5 | 0.209 | 4.2 | 2083.2 ± 18.6 | 43.5 | 0.381 | 7.2 | 2461.2 ± 22.9 | 47.3 | 0.450 | 6.7 |
| Exoskeletonsa | 582 ± 292 | 643 ± 2725 | 462 ± 270 | 305 ± 169.2 | ||||||||||||
NP, not present. Most unidentified copepods were copepodite stages.
aExoskeletons are not included in estimates of copepod or total zooplankton abundance.
The cyclopoid, Oithona spp. (consisting mostly of Oithona colcarva, but also Oithona plumifera and at least one other occasional Oithona sp.) were the next most abundant copepod type at all depths (Table VI, Fig. 2C). Oithona abundances were not affected by time of day (P = 0.472), but were significantly higher at 2.0 mab and surface samples than in substrate or 0.5 mab (P = 0.028; Table III).
Calanoid copepods were always present, but particularly prevalent in 2.0 mab and surface water samples. Acartia spp. were the most abundant calanoid spp., especially in some samples, which contained large numbers of Acartia danae and Acartia spinata (Table VI; Fig. 2E). Acartia spp. were more abundant at heights just above reef surfaces (0.5 and 2.0 mab) than at either the surface or the substrate (P = 0.004), and significantly less common during day time (12.00 and 18.00 h) samples (P < 0.001; Table III). This result matched field observations of Acartia swarming behaviors at about 2.0 mab (see Discussion). With the exception of Acartia spp., most calanoids were significantly more abundant in surface samples than closer to reef surfaces. Calanus spp. had a significant pattern of decreasing abundance with depth, with maximal densities in surface waters (P < 0.001; Fig. 2F). Calanus was also less abundant toward the end of the day when compared with night and early morning (12.00, 18.00 and 21.00 h) (Fig. 2F). Temora spp. (T. stylifera and T. turbinata) was significantly more abundant at depths above 0.5 mab, with the maximal densities in surface waters (Fig. 2G), but abundances did not differ by time of day (P = 0.888). Finally, Calocalanus spp. (most Calocalanus pavo) followed the trend of having greater abundances at the surface, yet this difference was not significant, and there were no differences with time (Fig. 2H). Other calanoid copepods including Paracalanus (mostly P. aculeatus) and Calanopia (mostly C. americana) also showed no significant differences with depth, but all had significant differences with time, both being less abundant during the day (Table III; graph not shown). Paracalanus results should be interpreted cautiously because of a depth × time interaction (P = 0.011, Table III). Several other calanoid groups were present but not abundant enough to determine patterns of occurrence.
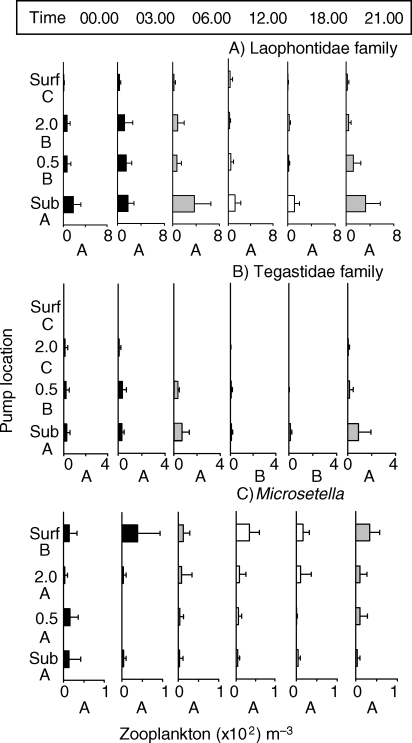
Harpacticoids are generally reef-associated or demersal, maintaining positions near reefs (Gheerardyn et al., 2008). As a group, harpacticoid abundances were significantly affected by both depth (P ≤ 0.000) and time (P = 0.011) (Table III; Fig. 3). The most abundant harpacticoids belonged to the Laophontidae and Tegastidae families. Laophontid harpacticoid copepods were absent from, or rarely seen in, surface samples, yet always present near the substrate (Fig. 3A). Tegastidae family harpacticoids, which are known to be coral parasites, were not present at the surface, and also had greatest densities near the substrate (P < 0.001). The Tegastidae harpacticoids also had significantly lower abundances during daytime samples (12.00 and 18.00 h) (P < 0.001; Fig. 3C). Conversely, Microsetella spp. (mostly M. rosea but also M. norvegica) were the only harpacticoids that were consistently more common in surface samples than at depth (P < 0.001; Fig. 3C; Table III). Microsetella is often associated with the pelagic nitrogen fixing cyanobacteria, Tricodesmium. Macrosetella spp. (mostly M. gracilis) were always more common in the two night samples (P ≤ 0.001), but evenly distributed by depth (P = 0.629) (Table III, graph not shown).
Fig. 3.
Abundance (mean × 102 ± 1 SD) of the three most abundant harpacticoid copepod genera. Graph details as for Fig. 2. Note differences in x-axis scale for each panel.
Several types of poecilostomatoids were routinely found in our samples (Table VI) with Corycaeus spp. being the most abundant. Corycaeus abundances did not differ by time (P = 0.132), but did have highly significant patterns of increasing abundances moving away from the substrate (P < 0.001, Table III, Fig. 2D). Oncaea abundances (mostly O. venusta and O. mediterranea) did not differ by height in the water column (P = 0.189) but did have a significantly lower abundance during the daytime (P = 0.025, Table III, graph not shown). Copilia spp., unlike other poecilostomatoids, were only found near reef surfaces (less than 2.0 mab) and were significantly more abundant at the substrate (P = <0.001) and at night (P = 0.003) (Tables III and Table VI; graph not shown).
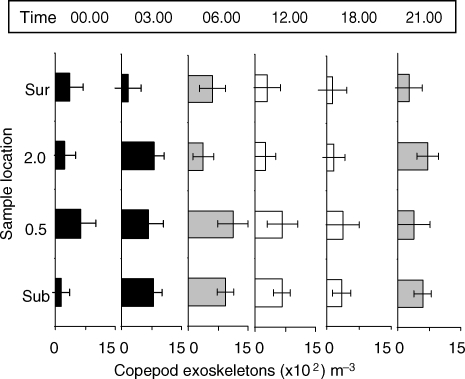
Copepod exoskeletons were present in every sample and very abundant in some, but were not included in calculations of zooplankton abundances. Exoskeletons were empty or nearly empty and had mostly undamaged setae and appendages. While not significant, there was a trend for higher abundances near reef surfaces when compared with water column surface (Table VI; Fig. 4).
Fig. 4.
Abundance (mean × 102 ± 1 SD) of copepod exoskeletons. Graph details as for Fig. 2.
Other common taxa
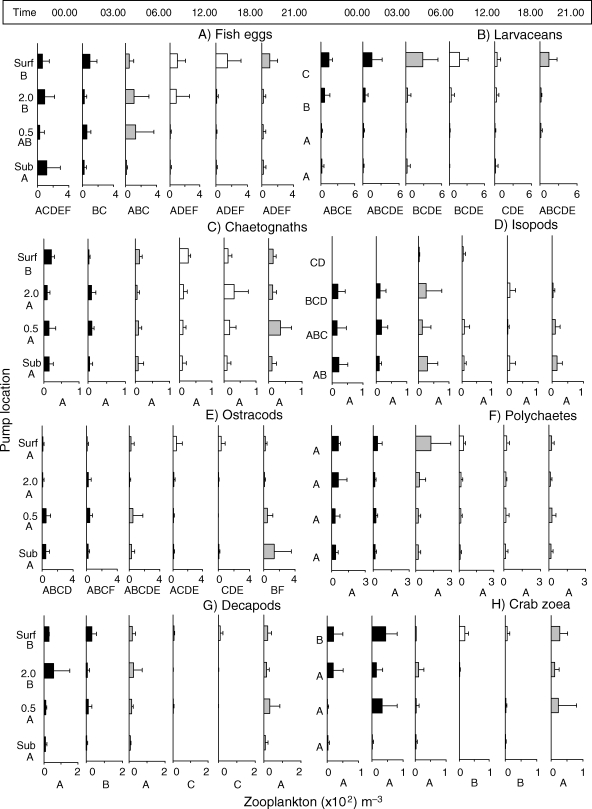
Fish eggs were routinely seen in our samples and varied by both time (P < 0.001; F = 5.447) and depth (P < 0.001; F = 171.97; Table V; Fig. 5). Eggs were significantly more abundant at night and in early morning hours than during the day and in the water column above 0.5 mab.
Fig. 5.
Abundance (mean × 102 ± 1 SD) of abundant non-copepod taxa. Graph details as for Fig. 2. Note differences in x-axis scale for each panel.
Zooplankton assemblages were also evaluated without copepods. Non-copepod taxa abundances significantly differed by depth. These groups showed greatest abundance at the surface, with no difference between the other three depths (P = 0.003). Larvaceans, a holoplanktonic taxon, were the most abundant non-copepod taxon. As expected, depth significantly affected distribution (P < 0.001), with a pattern of higher numbers as we sampled away from the substrate (Fig. 5B). Larvaceans were most abundant near sunrise and least abundant in late afternoon (Fig. 5B). Chaetognaths, a common holoplanktonic group, were evenly distributed by both depth (0.073) and time (P = 0.384; Fig. 5C).
Isopods were significantly more abundant near the substrate and at 0.5 mab, than further off the reef (P = 0.005). They were rarely seen in surface waters. Surprisingly, since they are often classified as demersal, we did not see significant differences over time (P = 0.296; Fig. 5D). Ostracods were found at all depths equally (P = 0.148), although there was a trend of higher abundances near the bottom. The species of ostracod in surface samples were different to those near the substrate, and this may have masked results for depth comparisons for the near reef species. Ostracod densities were lowest during the day and higher at sunset through sunrise, as expected for demersal forms (Table III, Fig. 5E). Polychaete abundances were evenly distributed by depth (P = 0.084) and throughout the diel cycle (P = 0.110), but there was a noticeable trend of peaks in abundance at night and at sunrise (Table III, Fig. 5F). An unusually large number of polychaetes were seen in one sample at the surface at time 06.00 (data not shown).
Finally, total decapods (Fig. 5G) followed the patterns of significantly low densities during the day with a migration up into the water column at sunset and throughout the night until dawn (P = <0.001; Fig. 5G). Decapods were significantly more abundant at 2.0 mab and surface waters than closer to reef structures (P = 0.003). A subset of the decapods, the brachyuran crab larvae (mostly Portunidea subfamily) included pre-zoea, zoea, and megalops stages (Fig. 5H). Patterns of distribution generally mimicked decapod larvae, but this effect should be viewed cautiously due to a depth by time interaction.
Average abundances for rare groups are reported by depth in Tables V and VI but not statistically compared.
Zooplankton biomass
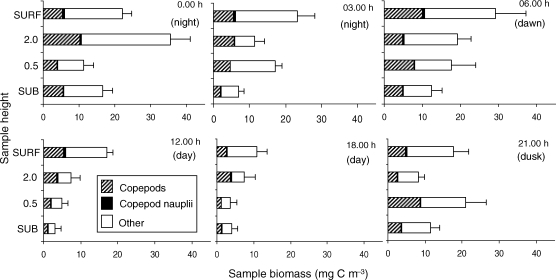
Average daily zooplankton biomass ranged from 8.1 to 21.4 mg C m−3. However, a more ecologically useful way to compare biomass is by height off the bottom or by time of day. Average biomass by height and by grouping of times into day, dawn/dusk and night is presented (Table IV; Fig. 6). Individual taxa biomass is also reported in Tables V and VI. While copepods (including naupliar stages) were the numerically dominant organisms, their contribution to biomass was, on average, only 35% of the total biomass (Table V). Biomass contributions by the less abundant, but larger organisms, were often quite important (Fig. 6).
Fig. 6.
Biomass (mg C m−3) of zooplankton (mean ± 1 SD) at each depth and time throughout the sampling period. Percent composition shown for copepods (hashed), copepod nauplii (black) and all other zooplankton taxa (open).
Biomass was significantly affected by both time (P = 0.012) and by height off the bottom (P = 0.031; two-factor repeated measures ANOVA). Further, when the six sampling times were grouped into day, dusk/dawn and night (two samples each), the average biomass was 18.0 ± 4.7 SD for night, 17.1 ± 3.5 SD, for dusk/dawn and only 7.3 ± 1.2 SD for daytime samples. Average biomass was not significantly different between night and dusk/dawn, but was different from the daytime samples (Table IV). Average biomass by day, dawn/dusk and night is also reported by height off the bottom (Table IV, Fig. 6). A two-factor repeated measures ANOVA analysis showed that the copepod contribution to biomass was equal at different heights off the bottom (P = 0.16), but varied with time (P = 0.04).
DISCUSSION
Total zooplankton densities at Conch Reef of 4396 ± 1949 SD are generally higher than most other studies reviewed by Heidelberg (Heidelberg et al., 2004), but comparable to other regions such as Discovery Bay, Jamaica (Heidelberg et al., 2004) and Tioman Island, Malaysia (Nakajima et al., 2008). It is not unusual that zooplankton abundances differ by location; however, our sampling strategy of using pumps with an intake fast enough to capture strong swimming zooplankton (Sebens et al., 1996) and the use of a 40 µm collection net, which retains numerically important naupliar stages helps explain the higher densities we observed. Developmental stages, such as nauplii, may be very important prey sources for heterotrophs. Other studies using larger mesh sizes or other collection methods such as towed nets or traps have likely underestimated smaller forms such as copepod nauplii and copepodite stages (Hopcroft et al., 1998; Paffenhofer and Mazzocchi, 2003). Higher average densities at this reef could also reflect a more eutrophic reef system than many found in more tropical latitudes.
Like others (Holzman et al., 2005; Yahel et al., 2005a), we found evidence for depletion of zooplankton in bottom waters (between substrate and 2.0 mab), most likely resulting from intense predation and clear evidence for surface water enrichment by zooplankton (Alldredge and King, 2009). During the daytime, planktivorous fishes provide significant predation pressure on zooplankton throughout the water column, although feeding occurs mainly within several meters of the reef (Hobson and Chess, 1978; Hamner et al., 1988; Motro et al., 2005). At night, predation pressure switches from mainly fish to nocturnal suspension feeders, such as corals. Coincident with this switch is also the emergence of demersal zooplankton into the water column (e.g. Emery, 1968; Holzman et al., 2005).
Copepods
Copepods were the numerically dominant taxa, ranging from 93 to 96% of the taxa at all depths (Table V), with naupliar stages comprising 35–44% of the total zooplankton assemblage in this study (Table VI). Interestingly, we found significantly fewer numbers of nauplii at the substrate and 0.5 mab than at 2.0 mab and near surface samples (Fig. 2b). We suggest that this is likely due to higher rates of predation near the substrate. While late stage copepods have the ability to position themselves in the water or avoid shears associated with boundary layers (Haury et al., 1980), naupliar stages are not strong swimmers and behave more as passive particles in flows commonly seen on reefs (Heidelberg et al., 1997). Contact rates with coral feeding structures are higher for nauplii than for other taxa, but some corals and other common zooplanktivores do not frequently capture this size class (Johnson and Sebens, 1993; Sebens et al., 1996, 1998) due to the small size not eliciting a capture response in the coral (Heidelberg et al., 1997). However, nauplii are subject to intense predation by other benthic feeders that can capture smaller particles, such as octocorals (Ribes et al., 1998) and larval fish (Buskey et al., 1996).
The most common cyclopoids (Oithona colcarva, O. plumifera) and calanoids (Acartia spp.) made up 41% of the total zooplankton at the substrate. The abundance of these two genera near the substrate, in this study, was up to a hundred times that of the most prevalent demersal taxa. Oithona spp., in particular, are available in large numbers as potential prey near reef surfaces throughout the diel cycle (Fig. 2C). While it is well known that copepods contribute to gut contents of planktivorous fishes and corals, each type is not represented equally based on availability (Hobson and Chess, 1976; Hobson and Chess, 1978; Hamner et al., 1988; Noda et al., 1992; Sebens et al., 1996, Sebens et al., 1998). Copepods exhibit strong escape behaviors that decrease probability of capture (Trager et al., 1994; Heidelberg et al., 1997), but these abilities can differ by copepod type. Differences in escape behaviors could contribute to differences in capture and depletion rates near reef surfaces and should be explored further.
The holoplanktonic calanoid, Acartia spp. exhibited a distribution unlike that of any other abundant copepod. Acartia was found in greatest abundance at 0.5 and 2.0 mab and higher in nocturnal samples. This result can be somewhat explained by what is known of the supply and potential behavior of Acartia danae at Conch Reef. Tidal bores have been shown to bring periodic high densities of Acartia danae (≤3900 m−3) to this reef (Leichter et al., 1998), and we observed occasional bores during our study, characterized by cooler temperatures, increased flows and an Acartia sp. dominated zooplankton assemblage that differed from that which occurs during non-bore periods (data in preparation). The normal vertical migration patterns for A. danae would be disrupted substantially when the species is swept over shallow reefs by processes such as internal bores. Once on the reef, Acartia were observed to form dense swarms about 2.0 mab, especially during the night. Acartia swarming behaviors have been observed before (Hamner and Carleton, 1979; Ambler et al., 1991; Buskey et al., 1996; Genin et al., 2005), with swarms up to 2 m in diameter and densities of up to 500 000 m−3. The bottom edge of these swarms typically occurs from 5.0 to 1.0 m away from the substrate (Hamner and Carleton, 1979; Ueda et al., 1983). The swarming behavior may be a result of a disrupted deeper offshore migration pattern or may be a mechanism to avoid predation (Emery, 1968). Swarming behaviors during the night with dispersal throughout the water column and daytime predation help explain the overall abundance maximum at 0.5 to 2.0 mab, and also the decreased average numbers during the day (Fig. 2E).
Other zooplankton
The contribution of pelagic holoplankton to reef ecosystems can be significant (Heidelberg et al., 2004), and many benthic reef zooplanktivores consume significant amounts of holoplankton (Porter, 1974; Lewis and Boers, 1991; Johnson and Sebens, 1993). Knowing the contribution of various holoplanktonic taxa, especially near reef surfaces where predation is most intense, provides essential information for determining their relative importance to reef ecosystems. Holoplankton in this study comprised up to 60% of nocturnal zooplankton within a few centimeter of reef surfaces in Jamaica (Heidelberg et al., 2004), and they frequently contaminated unsealed demersal traps (Robichaux et al., 1981). These organisms can be swept into coastal areas by oceanic currents (Hopkins et al., 1981; Yoshioka et al., 1985; Suarez-Morales and Gasca, 2000), by internal waves (Leichter et al., 1998, see above) through vertical mixing (Lagadeuc et al., 1997) and by wind generated breaking waves (Genovese and Witman, 2004). The most abundant non-copepod holoplanktonic zooplankton were larvaceans (appendicularians) (Fig. 5B). Open ocean larvacean abundances show no discernable day/night differences (Steinberg, 2008). However, when present over reefs, larvaceans are heavily preyed upon by planktivorous fishes (Hamner et al., 1988), suggesting that predation is controlling abundances and explaining the patterns observed in this study. Another holoplanktonic species, chaetognaths, showed no evidence of depletion in near-bottom areas. Unlike larvaceans, chaetognaths have strong swimming and escape responses and may not be subjected to intense predation (Heidelberg et al., 1997).
Unlike holoplankton that are swept onto reefs, demersal taxa are reef-residents that generally migrate up into the water column at dusk and return to the benthos at dawn. These groups and their migrations can be highly variable from night to night with multiple influences including, presence of predators (Ohman et al., 1983), nutritional factors (Sekino and Yamamura, 1999) and reproductive cycle status (Bollens and Frost, 1991). Decapods and brachyuran crab zoea were more abundant at night and in surface waters (Fig. 5G–H). Given the relatively strong and directed swimming abilities of these taxa, it is unclear if higher abundances in surface waters are due to active migration or if there is depletion in bottom waters through predation. Decapod larvae have been shown to be important prey for nocturnal planktivorous fishes (Hobson and Chess, 1978) and for corals (Sebens et al., 1996, 1998), suggesting predation as a significant cause of bottom water depletion.
Zooplankton biomass
Zooplankton biomass significantly increased over the reef during periods around dusk, dawn and night corresponding to a time of cessation of active fish predation and the emergence of demersal plankton and active behavioral positioning of plankton above reef structures. These are similar to conclusions drawn by others (Yahel et al., 2005b). Our range of zooplankton biomass near the substrate (nocturnal 7.0–16.7; dusk/dawn 11.5–12.4; day 3.1–3.9 mg C m−3) is higher than nocturnal, near-substrate biomass values at Discovery Bay, Jamaica (4.5 mg C m−3) (Heidelberg et al., 2004) and much higher compared to some other measurements of biomass over reefs (Roman et al., 1990; Yahel et al., 2005b). However, an evaluation of community composition between this study and Discovery Bay shows that Conch Reef (this study) has higher densities (proportions) of larger zooplankton (e.g. decapods) than Discovery Bay, which significantly affect biomass. The occurrence of periodic internal bores also provides a source of additional nutrients and particles that supports higher productivity on this reef (Leichter et al., 1998).
Factors causing zooplankton assemblage distribution patterns
The two biggest potential factors that can cause differing patterns of depletion in zooplankton communities are predation by a variety of zooplanktivores and zooplankton behaviors resulting in avoidance of reef surfaces. Like others (Yahel et al., 2005a), we saw patterns of decreasing abundances near reef surfaces for total zooplankton assemblages and many groups. Zooplankton feeding by benthic or by mobile predators is common on reefs. Corals rely on zooplankton as an important nutrient and energy source that supplements the contributions from zooxanthellate symbionts (Goreau et al., 1971; Sorokin, 1973; Porter, 1974; Sebens et al., 1996, 1998; Titlyanov et al., 2000, Ferrièr-Pages et al., 2003). Many reef fish also rely on zooplankton as a primary food source (Tranter and George, 1972; Glynn, 1973; Hobson and Chess, 1976; Hobson and Chess, 1978; Motro et al., 2005; Yahel et al., 2005a). Moreover, predation pressures exerted by planktivorous fish are far greater in areas up to 1.5 m above reef surfaces than higher in the water column where foraging behaviors can be restricted by piscivores in areas further off the reef (Motro et al., 2005).
One line of compelling evidence in our study that supports predation as a zooplankton community modifier is the occurrence of often numerous copepod exoskeletons. Copepod exoskeletons in good condition are egested by post-settling fish larvae and crionoids (Genin et al., 1995), by euphausiids (Haury et al., 1995) and by anemones and scleractinian corals (Purcell and Heidelberg, unpublished data). Exoskeletons have not been frequently reported as part of reef zooplankton data sets, although at times they can be very abundant. Genin et al. (Genin et al., 1995) were the first to carefully evaluate patterns of exoskeletons on a coral reef near Eilat, Israel. In their study, clear patterns of copepod exoskeleton abundance correlated to live zooplankton abundances, suggesting heavy zooplanktivores predation was the cause. We found greater numbers of exoskeletons in samples closest to the reef surfaces (Near substrate, 0.5 and 2.0 mab versus surface), where predation is likely to be most intense (Fig. 4). Interestingly, live copepods also showed clear trends of depletion in abundances near reef surfaces (Table V), possibly due to predation, as well.
Zooplankton swimming behaviors can also structure communities. Many reef zooplankton exhibit active behaviors that control their temporal and spatial location in the water column as discussed previously. Conversely, fish eggs, which do not have active swimming behaviors, had a pronounced depletion in bottom waters (Fig. 5A) and significantly higher abundances at night. The shift in abundance at sunset corresponds with our observations and those of Yahel et al., who suggested that many reef fish spawn around sunset, possibly to prevent visual predation (Yahel et al., 2005b). Fish eggs may also be advected onto reefs in open ocean surface layers.
The detailed description of temporal and spatial patterns of zooplankton community assemblages, especially close to reef surfaces, is a major contribution of this study. Our results will help refine models of zooplankton availability for benthic suspension feeders, such as corals and anemones, and for fish and other zooplanktivores on and just above reef surfaces. The use of detailed data on zooplankton community structure will facilitate better mathematical models evaluating potential sources and contribution of zooplankton to reef communities and for specific predators on reefs. Conversely, in the face of heavy predation pressure typically seen on reefs (e.g. Tranter and George, 1969; Sebens et al., 1998), these data may shed light on zooplankton habitat selection strategies in time and space that could be essential for the survival of certain types of zooplankton.
FUNDING
The project was supported by awards 2000-9924b from the University of North Carolina, Wilmington, National Undersea Research Program, NSF award OCE-9811577, and a U. Maryland Department of Biology award for honors undergraduate research to K.O.
ACKNOWLEDGEMENTS
We thank the many people who contributed to this project. J. Thomason (U. Newcastle) was a saturation mission collaborator and assisted with the zooplankton sampling effort. We thank S. Miller, C. Cooper, O. Rutten and the entire 2000 UNCW/NURC staff for logistic support. P. Thomason and S. Walker (U. Newcastle) and E. Haberkern (U. Maryland) provided mission surface support. Jim Liechter (UCSD) lent us an acoustic-Doppler velocimeters for field calibration tests of pumps. A. Willey (U. Maryland) and Matthew Mills (Stanford) provided assistance with zooplankton sample analyses. Robert Cerrato (SUNY, Stoneybrook) provided valuable assistance with statistical analyses. Zooplankton samples are maintained in the lab of K.P.S. at University of Washington Friday Harbor Laboratories.
REFERENCES
- Alldredge A. L., King J. M. Distribution, abundance, and substrate preferences of demersal reef zooplankton at Lizard Island Lagoon, Great Barrier Reef. Mar. Biol. 1977;41:317–333. [Google Scholar]
- Alldredge A. L., King J. M. The distance demersal zooplankton migrate above the benthos: implications for predation. Mar. Biol. 1985;84:253–260. [Google Scholar]
- Alldredge A. L., King J. M. Near-surface enrichment of zooplankton over a shallow back reef: implications for coral reef food webs. Coral Reefs. 2009 doi: 10.1007/s00338-009-0534-4. [Google Scholar]
- Ambler J. W., Ferrari F. D., Fornshell J. A. Population structure and swarm formation of the cyclopoid copepod Diothona oculata near mangrove cays. J. Plankton Res. 1991;13:1257–1272. [Google Scholar]
- Anderson T. W. An Introduction to Multivariate Statistical Analysis. Wiley; 1958. [Google Scholar]
- Aronson R. B., Edmunds P. J., Precht W. F., et al. Large-scale, long-term monitoring of Caribbean coral reefs: simple, quick, inexpensive techniques. Atoll. Res. Bull. 1994;42:1–19. [Google Scholar]
- Bollens S. M., Frost B. W. Diel vertical migration in zooplankton: rapid individual response to predators. J. Plankton Res. 1991;13:1359–1365. [Google Scholar]
- Buskey E. J., Peterson J. O., Ambler J. W. The swarming behavior of the copepod Dioithona oculata: in situ and laboratory studies. Limnol. Oceanogr. 1996;41:513–521. [Google Scholar]
- Emery A. R. Preliminary observations on coral reef plankton. Limnol. Oceanogr. 1968;13:293–303. [Google Scholar]
- Ferrièr-Pages C., Witting J., Sebens K. P. Effect of natural zooplankton feeding on the tissue and skeletal growth of the scleractinian coral Stylophora pistillata. Coral Reefs. 2003;22:229–240. [Google Scholar]
- Genin A., Gal G., Haury L. Copepod carcasses in the ocean. II. Near coral reefs. Mar. Ecol. Prog. Ser. 1995;123:65–71. [Google Scholar]
- Genin A., Jaffe J. S., Reef R., et al. Swimming against the flow: a mechanism of zooplankton aggregation. Science. 2005;308:860–862. doi: 10.1126/science.1107834. [DOI] [PubMed] [Google Scholar]
- Genovese S. J., Witman J. D. Wind-mediated diel variation in flow speed in a Jamaican back reef environment: effects on ecological processes. Bull. Mar. Sci. 2004;75:281–293. [Google Scholar]
- Gheerardyn H., De Troch M., Ndaro S. G. M., et al. Community structure and microhabitat preferences of harpacticoid copepods in a tropical reef lagoon (Zanzibar Island, Tanzania) J. Mar. Biol. Assoc. UK. 2008;88:747–758. [Google Scholar]
- Glynn P. W. Ecology of a Caribbean coral reef. The Porites reef-flat biotope: Part II. Plankton community with evidence for depletion. Mar. Biol. 1973;22:1–21. [Google Scholar]
- Goreau T. F., Goreau T. I., Yonge C. M. Reef corals: autotrophs or heterotrophs? Biol. Bull. Mar. Biol. Lab. Woods Hole. 1971;141:247–260. [Google Scholar]
- Hammer R. M. Day-night differences in the emergence of demersal zooplankton from a sand substrate in a kelp forest. Mar. Biol. 1981;62:275–280. [Google Scholar]
- Hamner W. M., Carleton J. H. Copepod swarms: attributes and role in coral reef ecosystems. Limnol. Oceanogr. 1979;24:1–14. [Google Scholar]
- Hamner W. M., Jones M. S., Carleton J. H., et al. Zooplankton, planktivorous fish, and water currents on a windward reef face: Great Barrier Reef, Australia. Bull. Mar. Sci. 1988;42:459–479. [Google Scholar]
- Haury L., Fey C., Gal G., et al. Copepod carcasses in the Ocean. I. Over seamounts. Mar. Ecol. Prog. Ser. 1995;123:57–63. [Google Scholar]
- Haury L. R., Kenyon D. E., Brooks J. R. Experimental evaluation of the avoidance reaction of Calanus finmarchicus. J. Plankton Res. 1980;2:187–202. [Google Scholar]
- Heidelberg K. B., Sebens K. P., Purcell J. E. Effects of prey escape and water flow on feeding by the scleractinian coral Meandrina meandrites. Proceedings of Eighth International Coral Reef Symposium. 1997:1081–1086. [Google Scholar]
- Heidelberg K. B., Sebens K. P., Purcell J. E. Composition and sources of near reef zooplankton on a Jamaican forereef along with implications for coral feeding. Coral Reefs. 2004;23:263–276. [Google Scholar]
- Hobson E. S., Chess J. R. Trophic interactions among fishes and zooplankters near shore at Santa Catalina Island, California. Fish. Bull. 1976;74:567–598. [Google Scholar]
- Hobson E. S., Chess J. R. Trophic relations among fishes and plankton in the lagoon at Enewetak Atoll, Marshall Islands. Fish. Bull. 1978;76:133–153. [Google Scholar]
- Holzman R., Reidenbach M. A., Monismith S. G., et al. Near-bottom depletion of zooplankton over a coral reef II: relationships with zooplankton swimming ability. Coral Reefs. 2005;24:87–94. [Google Scholar]
- Hopcroft R. R., Roff J. C., Lombard D. Production of tropical copepods in Kingston Harbor, Jamaica: the importance of small species. Mar. Biol. 1998;130:593–604. [Google Scholar]
- Hopkins T. L., Milliken D. M., Bell L. M., et al. The landward distribution of oceanic plankton and micronekton over the west Florida continental shelf as related to their vertical distribution. J. Plank. Res. 1981;3:645–658. [Google Scholar]
- Huys R., Boxshall G. The orders of copepods. In: Huys R., Boxshall G. A., editors. Copepod Evolution. The Ray Society Series. vol. 159. London, UK: Ray Society; 1991. pp. 31–314. [Google Scholar]
- Johnson A. S., Sebens K. P. Consequences of a flattened morphology: effect of flow on feeding rates of the scleractinian coral Meandrina meandrites. Mar. Ecol. Prog. Ser. 1993;99:99–114. [Google Scholar]
- Lagadeuc Y., Boule M., Dodson J. J. Effect of vertical mixing on the vertical distribution of copepods in coastal waters. J. Plankton Res. 1997;19:1183–1204. [Google Scholar]
- Leichter J. J., Wing S. R., Miller S. L., et al. Pulsed delivery of subthermocline water to Conch Reef (Florida Keys) by internal tidal bores. Limnol. Oceanogr. 1996;41:1490–1501. [Google Scholar]
- Leichter J. J., Shellenbarger G., Genovese S. J., et al. Breaking internal waves on a Florida (USA) coral reef: a plankton pump at work? Mar. Ecol. Prog. Ser. 1998;166:83–97. [Google Scholar]
- Leichter J. J., Stewart H. L., Miller S. L. Episodic nutrient transport to Florida coral reefs. Limnol. Oceanogr. 2003;48:1394–1407. [Google Scholar]
- Lewis J. B., Boers J. J. Patchiness and composition of coral reef demersal zooplankton. J. Plankton Res. 1991;13:1273–1289. [Google Scholar]
- Motro R., Ayalon I., Genin A. Near bottom depletion of zooplankton over coral reefs: III: vertical gradient of predation pressure. Coral Reefs. 2005;24:95–98. [Google Scholar]
- Nakajima R., Yoshida T., Othman B. H. R., Toda T. Diel variation of zooplankton in the tropical coral-reef water of Tioman Island, Malaysia. Aquat Ecol. 2008 doi: 10.1007/s10452-008-9208-5. [Google Scholar]
- Noda M., Kawabata K., Gushima K., et al. Importance of zooplankton patches in foraging ecology of the planktivorous reef fish Chromis chrysurus (Pomacentridae) at Kuchinoerabu Island, Japan. Mar. Ecol. Prog. Ser. 1992;87:251–263. [Google Scholar]
- Ohman M. D., Frost B. W., Cohen E. B. Reverse diel vertical migration: an escape from invertebrate predators. Science. 1983;220:1404–1406. doi: 10.1126/science.220.4604.1404. [DOI] [PubMed] [Google Scholar]
- Omori M., Ikeda Y. Methods in Marine Zooplankton Ecology. Wiley; 1984. [Google Scholar]
- Owre H. B., Foyo M. Copepods of the Florida Current. Institute of Marine Science, University of Miami; 1967. [Google Scholar]
- Paffenhofer G. A., Mazzocchi M. G. Vertical distribution of subtropical epiplanktonic copepods. J. Plankton Res. 2003;25:1139–1156. [Google Scholar]
- Porter J. W. Zooplankton feeding by the Caribbean reef-building coral Montastrea cavernosa. Proceeding of. Second International Coral Reef Symposium. 1974:111–125. [Google Scholar]
- Ribes M., Coma R., Gili J. -M. Heterotrophic Feeding by Gorgonian Corals with Symbiotic Zooxanthella. Limnol. Oceanogr. 1998;43:1170–1179. [Google Scholar]
- Robichaux D. M., Cohen A. C., Reaka M. L., et al. Experiments with zooplankton on coral reefs, or, will the real demersal plankton please come up? Mar. Ecol. 1981;2:77–94. [Google Scholar]
- Roman M. R., Furnas M. J., Mullin M. M. Zooplankton abundance and grazing at Davies Reef, Great Barrier Reef, Australia. Mar. Biol. 1990;105:73–82. [Google Scholar]
- Sebens K. P., Vandersall K. S., Savina L. A., et al. Zooplankton capture by two scleractinian corals, Madracis mirabilis and Montastrea cavernosa, in a field enclosure. Mar. Biol. 1996;127:303–317. [Google Scholar]
- Sebens K. P., Grace S. P., Helmuth B., et al. Water flow and prey capture by three scleractinian corals, Madracis mirabilis, Montastrea cavernosa, and Porites porites, in a field enclosure. Mar. Biol. 1998;131:347–360. [Google Scholar]
- Sekino T., Yamamura N. Diel vertical migration of zooplankton: optimum migrating schedule based on energy accumulation. Evol. Ecol. 1999;13:267–282. [Google Scholar]
- Shinn E. A., Lidz B. H., Kindinger J. L., et al. Reefs of Florida and the Dry Tortugas. A Guide to the Modern Carbonate Environments of the Florida Keys and the Dry Tortugas. St. Petersburg, Florida: USGS; 1989. [Google Scholar]
- Sorokin Y. I. On the feeding of some scleractinian corals with bacteria and dissolved organic matter. Limnol. Oceanogr. 1973;18:380–386. [Google Scholar]
- Steinberg D. K. A comparison of mesopelagic mesozooplankton community structure in the subtropical and subarctic North Pacific Ocean. Deep-Sea Res. II. 2008;55:1615–1635. [Google Scholar]
- Suarez-Morales E., Gasca R. Epipelagic copepod assemblages in the western Caribbean sea (1991) Crustaceana. 2000;73:1247–1257. [Google Scholar]
- Titlyanov E. A., Leletkin V. A., Dubinsky Z. Autotrophy and predation in the hermatypic coral Stylophora pistillata in different light habitats. Symbiosis. 2000;29:263–281. [Google Scholar]
- Trager G., Achituv Y., Genin A. Effects of prey escape ability, flow speed, and predator feeding mode on zooplankton capture by barnacles. Mar. Biol. 1994;120:251–259. [Google Scholar]
- Tranter D. J., George J. Coral reefs as biotypes: Invertebrates: Zooplankton abundance at Kavaratti and Kalpeni atolls in the Laccadives. In: Mukundan C., Gopinadha Pillai C. S., editors. Proceedings of 1st International Symposium on Corals and Coral Reefs. Mandapam, India.; June 1969; Cochin: Marine Biology Association India; 1969. pp. 239–256. [Google Scholar]
- Tranter D. J., George J. Zooplankton abundance at Kavaratti and Kalpeni Atolls in the Laccadaves. In: Mukundan C., Pillai C. S. G., editors. Proceeding of First International Coral Reef Symposium. 1972. pp. 239–256. [Google Scholar]
- Ueda H., Kuwahara A., Tanaka M., et al. Underwater observations on copepod swarms in temperate and subtropical waters. Mar. Ecol. Prog. Ser. 1983;11:165–171. [Google Scholar]
- Uye S. Length-weight relationships of important zooplankton from the inland Sea of Japan. J. Oceanogr. Soc. Jpn. 1982;38:149–158. [Google Scholar]
- Yahel R., Yahel G., Genin A. Near-bottom depletion of zooplankton over coral reefs: I: diurnal dynamics and size distribution. Coral Reefs. 2005;a 24:75–85. [Google Scholar]
- Yahel R., Yahel G., Berman T., et al. Diel patterns with abrupt crepuscular changes of zooplankton over a coral reef. Limnol. Oceanogr. 2005;b 503:930–944. [Google Scholar]
- Yoshioka P. M., Owen G. P., Pesante D. Spatial and temporal variations in Caribbean zooplankton near Puerto Rico. J. Plankton Res. 1985;7:733–751. [Google Scholar]