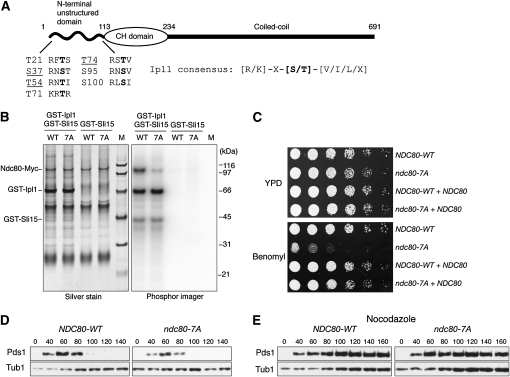
Figure 1.—
An N-terminal Ndc80 mutant lacking Ipl1 phosphorylation sites is benomyl sensitive but spindle checkpoint proficient. (A) A schematic of Ndc80 shows that it contains seven putative Ipl1 phosphorylation Ser/Thr sites (shown in boldface type) in the unstructured N terminus. Phosphorylation sites detected by MS are underlined. Minichromosomes were purified from cells expressing Gip4 from the galactose promoter (SBY6247) for 3 hr and subjected to MS analysis (Kim et al. 2007 and unpublished data). Yeast strains used in this study are listed in supporting information, Table S1. (B) Phosphorylation of the Ndc80-7A phospho-deficient mutant by Ipl1 is reduced in vitro. Ndc80-WT-Myc (WT, SBY7258) and Ndc80-7A-Myc (7A, SBY7259) proteins were immunoprecipitated with anti-Myc antibodies and used as substrates in vitro for an Ipl1 kinase assay as previously described (Buvelot et al. 2003). M, molecular weight markers. (C) Ndc80-7A mutant cells are viable, but show hypersensitivity to benomyl that is rescued by endogenous NDC80. Serial dilutions (fivefold) of NDC80-WT and ndc80-7A cells in the presence or absence of an endogenous NDC80 were plated on YPD plates with or without 7.5 μg/ml benomyl (SBY7258, SBY7259, SBY6859, and SBY6860). (D) Ndc80-7A cells do not exhibit defects in cell-cycle progression. Cells containing Pds1-Myc and either NDC80-WT (SBY7120) or ndc80-7A (SBY7123) were released from G1 and lysates were prepared at the indicated time points and monitored for Pds1-Myc by immunoblot as previously described (Biggins et al. 1999). (E) Ndc80-7A cells activate the spindle checkpoint in response to microtubule depolymerization. The experiment in D was repeated by releasing cells into 10 μg/ml nocodazole.