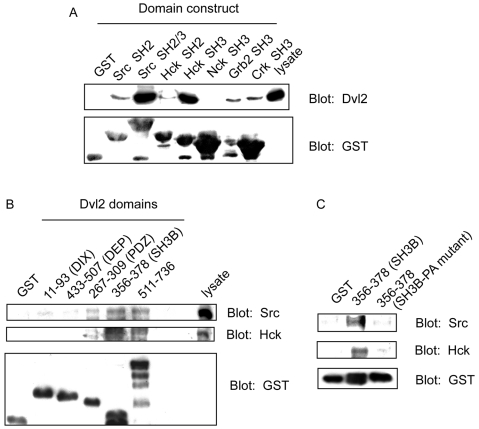
Fig. 3.
Src docking to Dvl2: analysis of potential docking domains. (A) Src SH2/SH3 and Hck SH3 domains display docking of Dvl2. Docking of Dvl2 to SH3 domains was analyzed by use of immobilized GST-SH3 domain fusion proteins. Cell lysates were incubated with each of the immobilized GST SH3 domains indicated as well as with GST itself (as a control) at 4°C for 1 hour. Bound proteins were analyzed by SDS-PAGE, blotted, and stained with either anti-Dvl2 (top panel) or anti-GST antibodies (bottom panel). Blots representative of three separate experiments are displayed. (B) Dvl2 domains dock Src and Hck tyrosine kinases. Cell lysates were incubated with an immobilized Dvl2 domain: 11-93 (DIX), 433-507 (DEP), 267-309 (PDZ), 356-378 (putative SH3 binding containing region, SH3B), 511-736 (C-terminus) and GST itself, at 4°C for 1 hour. Proteins docking to the Dvl2 domains were resolved by SDS-PAGE and analyzed by immunoblotting and staining with anti-Src, anti-Hck and anti-GST (loading control). Blots representative of four independent experiments are displayed. (C) Mutagenesis of the Dvl2 SH3-binding domain abolishes docking of Src and Hck. Cell lysates were incubated with either GST itself or GST-putative SH3-binding containing region (356-378, SH3B) or GST-mutant SH3 binding containing region (370-376, PxxPxxP to AxxAxxA in 356-378, SH3B-PA mutant). Bound proteins were analyzed by SDS-PAGE and immunoblotting, and stained with anti-Src, anti-Hck and anti-GST antibodies. Blots representative of three independent experiments are displayed.