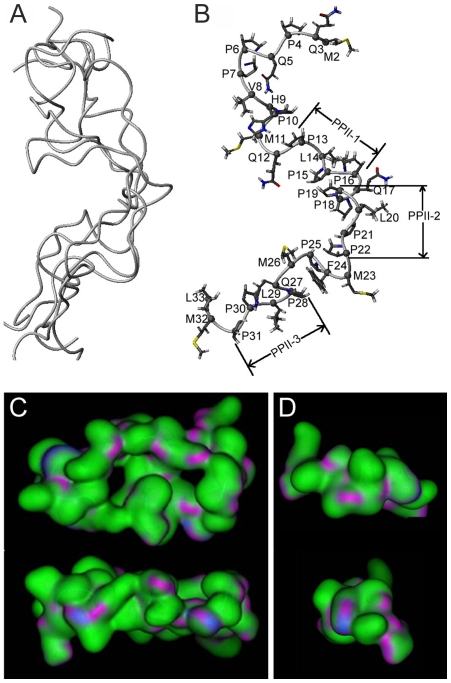
Figure 4. PXX33 atomic structure derived from solution NMR.
(A) The five lowest energy structures selected from 200 calculated structures represented in ribbon form. While the overall structure was similar between all five conformations, there was a greater variability at the N-terminal PXX12 region. (B) Backbone ribbon representation and side chain heteroatom representation of one PXX33 lowest energy structure. Three polyproline II helix regions—P13–P16 (PPII-1), P19–P22 (PPII-2), and P28–P31 (PPII-3) —are labeled. (C–D) Increase in surface area in larger PXX33 polypeptides (C) versus PXX12 polypeptides (D), resulting in increased interaction, van der Waals attraction, and denser aggregates.