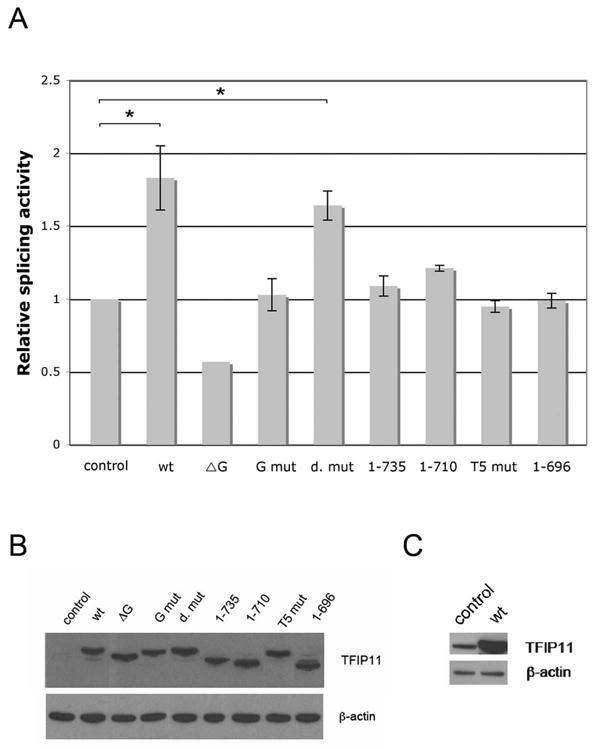
Figure 4. TFIP11 stimulates splicing activity in vivo.
A, TFIP-FLAG, pCMV-3Tag-8 (control) and a series of mutated TFIP11 constructs containing a C-terminus FLAG tag were cotransfected with double reporter plasmid pTN24 in HEK293 cells. The ratio of luciferase to β-galactosidase acitivities were calculated as relative splicing activity with the activity of the empty vector set at 1. The results shown here (with the exception of the ΔG vector where the experiment was repeated only once) represent three independent experiments in which three wells of cells were transfected and each cell lysate was measured in triplicate. Standard error of mean shown. Statistically significant comparison data for the wt and d. mut vectors, when compared to control, indicated by *, p < 0.05. B, Immunoblot analysis of cell lysates from pCMV-3Tag-8 (control), TFIP-FLAG (wt) and mutated TFIP11 constructs (ΔG, G mut, d. mut, 1–735, 1–710, T5 mut and 1–696) transfected cells using rabbit-generated antibody against TFIP11 and β–actin included as control. C, The band for the control (endogenous TFIP11; left column), compared to transfected TFIP-FLAG (wt; right column), became apparent when film exposed for a longer time.