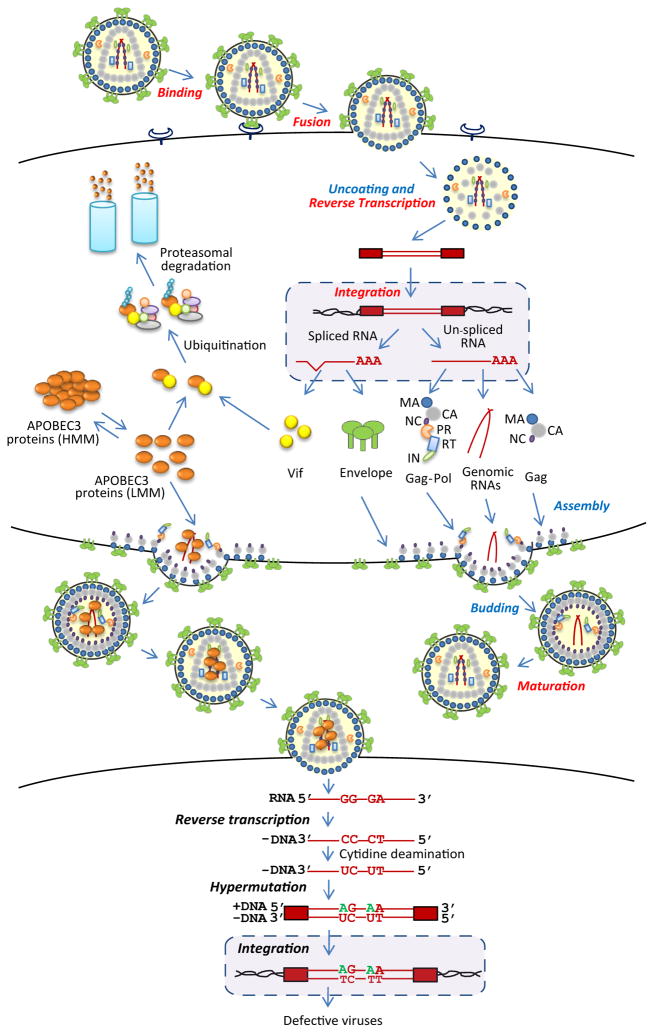
Figure 1.
HIV-1 replication and inhibition by APOBEC3 proteins. HIV-1 binds to cell surface receptors, triggering fusion of the viral and cellular membranes. Uncoating of the viral nucleoprotein complex, along with reverse transcription, results in the formation of double-stranded DNA, which is integrated into the host cell DNA to form a provirus. The proviral genome is transcribed into spliced and unspliced RNAs and translated to form viral structural proteins group antigen (Gag), Gag-polymerase (Gag-pol), and envelope (Env), along with the accessory proteins, including the virion infectivity factor (Vif). The viral genomic RNA and structural proteins are assembled and released from the plasma membrane to form immature viral particles; processing of the viral proteins by protease (PR) into their mature components, which include matrix (MA), capsid (CA), nucleocapsid (NC), PR, reverse transcriptase (RT), and integrase (IN), leads to the formation of the mature virion. Virion binding, fusion, reverse transcription, integration, and maturation (shown in red) are the targets of currently available antiviral drugs. APOBEC3 proteins exist in the cytoplasm in low molecular mass (LMM) complexes or RNA associated high molecular mass (HMM) complexes. In the absence of Vif, APOBEC3 proteins are incorporated into virion particles; Vif associates with the APOBEC3 proteins and targets them for ubiquitination and proteasomal degradation. In the infected cell, APOBEC3 proteins inhibit reverse transcription, catalyze cytidine deamination of the minus-strand DNA and G-to-A hypermutation, and inhibit viral DNA integration.