Fig. 1.
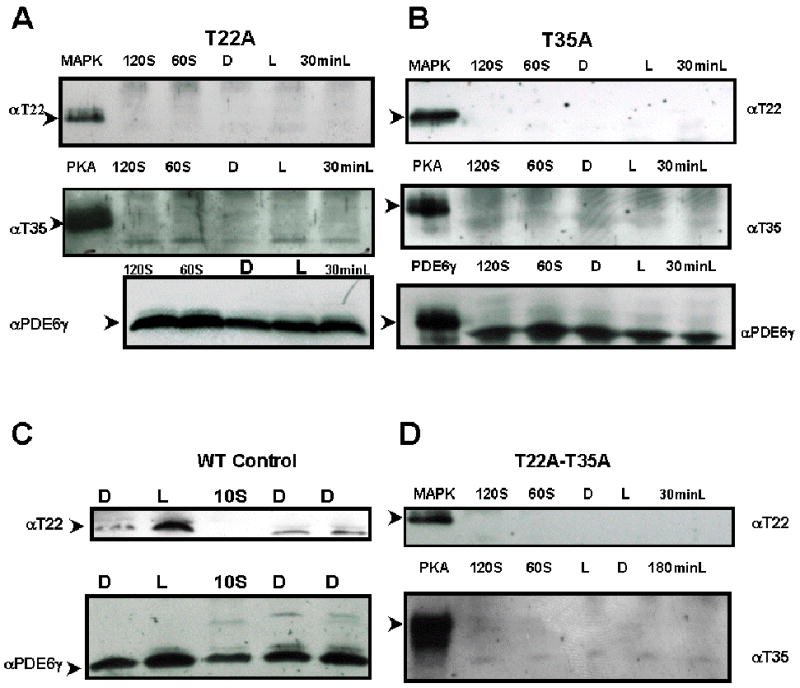
Both anti-pT22 and anti-pT35 antibodies recognized specific residues in phosphorylated PDE6γ.
Immunoblot of mutant transgenic retinal extracts probed with rabbit anti-phosphoT22 antibody recognizing the phosphorylated T22 residue of PDE6γ or rabbit anti-phosphoT35 antibody recognizing the phosphorylated T35 residue of PDE6.
MAPK, mitogen-activated protein kinase phosphorylated PDE6γ marked the expected molecular weight of pT22- PDE6γ at 11 kDa (top row, Fig 1A, B and D).
PKA, protein kinase A phosphorylated PDE6γ marked the expected molecular weight of pT35- PDE6γ (second row, Fig 1A, B and D).
Panel A: T22A transgenic retinal extract. T35 phosphorylation was lost in T22A transgenic rods.
Panel B: T35A transgenic retinal extract. Light induced T22 phosphorylation is lost in T35A transgenic rods.
Panel C: Control wild-type MF1 retinal extract. Light induced T22 phosphorylation was seen.
Panel D: T22A-T35A transgenic retinal extract. Both light-induced T22 and basal T35 phosphorylation was significantly diminished.
Presence of PDE6γ in each sample was confirmed by stripping and re-staining the filter with rabbit anti-native PDE6γ antibody. Reprobing with anti-PDE6γ antibody demonstrated expression of PDE6γ protein in these lysates.
D, overnight dark-adapted retinal extracts; L, light-adapted; 60S, 60 seconds of light exposure to a dark-adapted retina; 120S, 120 seconds of light exposure to a dark-adapted retina; 30minL, 30 minutes of light exposure to a dark-adapted retina; 180 minL, 180 minutes of light exposure to a dark-adapted retina. MAPK, mitogen-activated protein kinase phosphorylated PDE6; PKA, protein kinase A phosphorylated PDE6. Both pT22 and pT35 antibodies only recognized the phosphorylated cognate peptides and anti-pT22 only recognized MAP kinase-treated (phosphorylates T22), while anti-pT35 only recognized PKA kinase-treated (phosphorylates T35) recombinant PDE6γ.
