Abstract
We discovered B lymphocyte deficient mice within a group of B10.A-CD45.1 mice, and established that this deficiency was a recessively inherited trait. Gene mapping and sequence analysis showed a mutation in the 3rd exon of the Cd79b gene (c.224G>A) that leads to the generation of a stop codon (W75X) in the mutant mouse. FACS analysis of bone marrow cells showed that the mutant mice did not express the CD79B antigen. In order to establish where the block in development happens, we analyzed CD43posB220pos B lymphocyte precursors present in the mutant mice, and found that the fraction C′ (corresponding to early pre-B lymphocytes) was absent in the mutant mouse whereas fractions B and C showed a relative accumulation. As expected, we found no IgG or IgA in mutant mice. These results suggest that this CD79b mutant strain may be a useful tool for immunological research into human immunodeficiencies.
Introduction
Primary immunodeficiency manifested by agammaglobulinemia and absence of circulating B lymphocytes is the most common congenital immune deficiency1. Since 1993, when the first gene responsible for agammaglobulinemia was identified2; 3, knowledge of the genome and progress in DNA sequencing have facilitated the discovery of mutations in several other genes in patients with agammaglobulinemias 1. The most recently reported gene was CD79B coding for CD79B antigen (also known as Igβ) with mutations found in 2 patients4; 5.
Genetically manipulated mice are useful tools for elucidating mechanisms and testing treatment approaches for human diseases. One disadvantage, however, is the need to backcross for many generations to isolate the manipulated gene from other potential operators. Even after many generations, it is difficult to ensure that the observed phenotype is not the result of a linked locus. In the case of agammaglobulinemia, where patients mostly have hypomorphic point mutations, mouse models with similarly restricted defects would be the most ideal for the research.
In this report, we describe a novel mouse with a spontaneous mutation in the Cd79b gene leading to a block in B cell development and agammaglobulinemia.
Results & Discussion
While isolating organs from a group of B10.A-CD45.1 mice, we noticed that 2 mice out of 16 did not have Peyer’s Patches, and suspected that the absence of B lymphocytes might be the possible cause6; 7 and analyzed spleens of these mice for B220 positive cells. We found less than 0.5% B220pos cells, confirming that these 2 mice did not have peripheral B cells. Investigating further, we analyzed blood from other mice of the same line from the same vendor and found that 7 out of 30 of those mice did not have blood-borne B cells.
To determine whether this observation was the result of an inheritable mutation, (as opposed to, for example, a B-cell depleting virus), we crossed B cell negative mice to B cell positive mice of the same strain. 100% of the F1 pups had blood-borne B cells, while ~20% (10/51) of the F2 generation were negative, showing a clear recessive inheritance of the trait (Fig. 1a). As a complementation test, we also crossed the B cell negative mice to a strain of B cell negative mice that contained mutation in the Immunoglobulin locus (μMT; mutated in the 3′ end of the mu heavy chain), and found that 100% of the F1 progeny of both crosses contained B cells (not shown). Thus the new mutant was unlikely to be located within the Immunoglobulin locus.
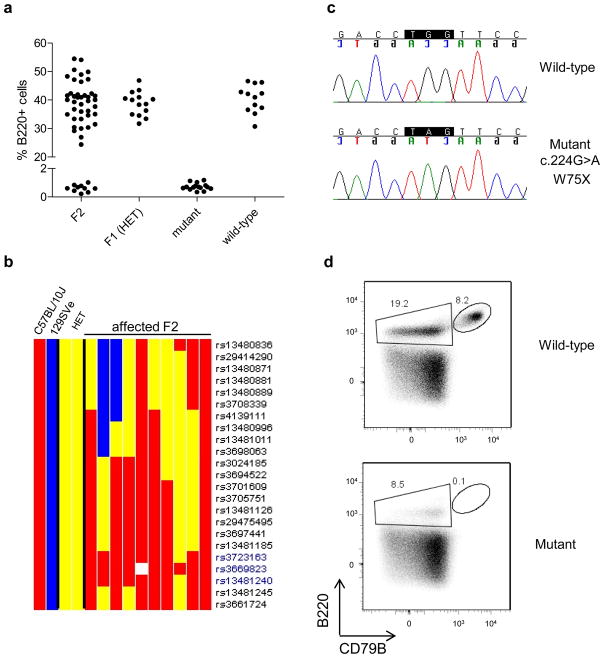
Figure 1. Finding the genetic defect in the spontaneous mutant mouse.
a) Percentage of B220pos positive cells in blood of wild type, mutant, F1 heterozygous (HET), and F2 mice.
b) Chromosome 11 map for SNP markers in B10 (red), 129SVE (Blue), HET – heterozygous (yellow), and F2 affected (absence of B220+ cells) mice. Technical failure to detect a SNP shown in white. The region between rs3723163 and rs13481240 was the only place in the genome where all of the affected F2 mice had SNPs inherited from the affected B10.A grandparent (red).
c) Chromatograms of the relevant portion of exon 3 of the Cd79b gene from the mutant and wild type mice.
d) Surface expression of the B220 and CD79B antigens on bone marrow cells of the mutant and wild type mice (gated on 7AAD negative cells).
Mice & Gene Mapping: Mice carrying the Cd79bW75X mutation were bred in the Taconic Farms NIAID contract facility. For gene mapping studies, (mutant x 129/SvEv)F1 mice were intercrossed to generate F2 progeny, which were screened for presence of B cells in peripheral blood. DNA samples from 10 affected F2 mice, two F1 mice, one 129SVE and one B10.A mouse (the background strain where the mutant mice were found) mouse were genotyped for strain-specific SNPs using the SNP array from Illumina (mouse low density linkage panel) by the Partners HealthCare Center for Personalized Genetic Medicine (PCPGM), Harvard Medical School. Cd79b gene and the promoter region were sequenced in the mutant (2 pool samples, each pool containing DNA samples from 2 individual mice) and B10.A-CD45.1 mice (one DNA pool sample containing samples from 5 individual mice). Experiments were approved by the NIAID Animal Care and Use Committee at NIH, which is accredited by the AALAC. The mutant mouse B10.Cg-PtprcaCd79bm1GhostH2a is registered in the MGI database under accession number 3829352.
FACS staining: Proteins expressed on the surface of blood and bone marrow cells were stained following standard protocols. The fluorochrome-conjugated antibodies were B220-PE (clone RA3-6B2; BD Pharmigen, San Jose, CA, USA), anti-CD79B-FITC (clone HM79b; BD Pharmigen, San Jose, CA, USA); 7-Aminoactinomycin D (7AAD; BD) was used to identify living cells. Data were acquired on a BD FACSCanto™ flow cytometer and analyzed using FlowJo software.
To identify the mutation responsible for the absence of B cells in the mutant mice, we crossed the B cell negative mice to normal mice of strain 129SVE and screened the F2 progeny for the presence of circulating B cells. As expected for a recessively inherited trait, approximately 25% (94/389) of the F2 mice were affected. Using strain-specific genetic markers, we found that the affected locus mapped to a 10 Mb region between rs3723163 and rs13481240 on chromosome 11 (Fig. 1b) that contained approximately 150 genes. Among those lay a gene (Cd79b) already known to be involved in B cell development, making this gene the best candidate for further investigation. After direct sequencing of the Cd79b gene 8 in the mutant and wild type mice, we indentified a mutation in exon 3 c.224G>A that leads to generation of a stop codon (TGG>TAG; W75X) in the mutant mouse (Figure 1c). The mutation was in the Ig-like domain of CD79b, which is responsible for binding of Igβ to Igα. The two molecules together constitute the signaling part of the B cell antigen-receptor complex, which is expressed throughout B cell development, from early B-cell precursors to plasma cells 9; 10.
As expected, FACS analysis of bone marrow cells showed that the mutant mice we did not express the CD79B antigen, whereas it was strongly expressed on B220pos cells in wild type mice (Figure 1d). We named the mutant mouse strain B10.Cg-PtprcaCd79bm1GhostH2a, in accordance with the Mouse Genome Informatics (MGI) formal nomenclature where Cg states for congenic. For general usage, synonyms B10.A-CD45.1-Cd79bmut or Cd79bmut can be utilized.
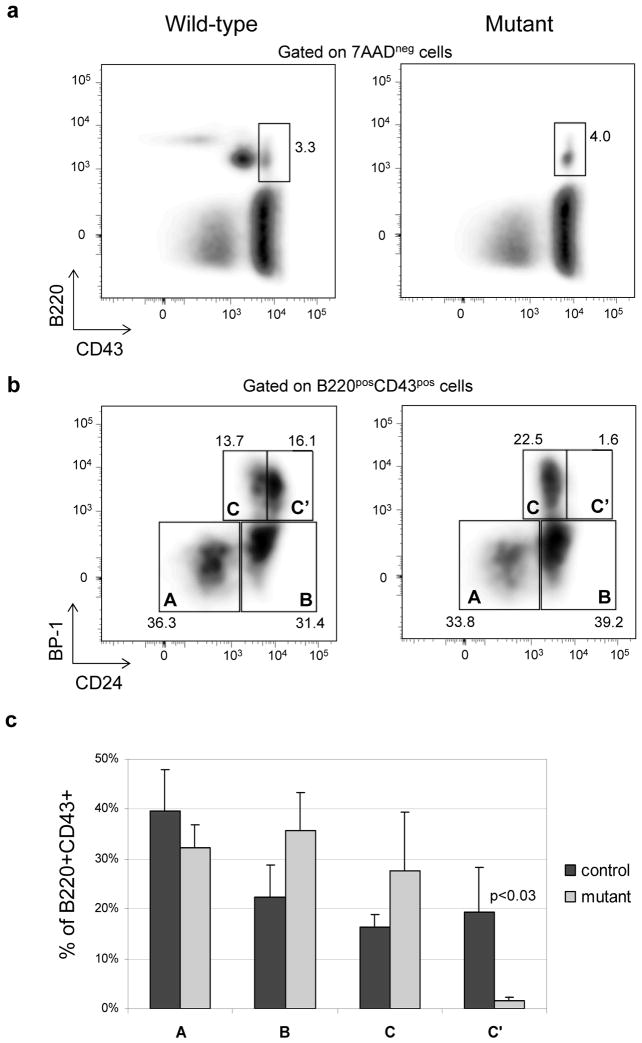
A knockout mouse for Cd79b has previously been generated and shown to have a complete block in B cell development at the CD43posB220pos stage11. This cell population includes different developmental stages, from pre-proB to early preB cells, that have been designated as fractions A, B, C and C′ 12. The authors did not find clear differences in the distributions of these fractions between Cd79b knockout and wild-type mice. However, because the early preB cells (fraction C′) are the first cells to appear after receiving a signal through IgM/Igβ/Igα complex that stimulates them to enter the cell cycle12, we surmised that these cells should be missing in the KO mouse (because the pre-BCR complex is not formed). Therefore, we analyzed the proportions of cells undergoing B cell development in the bone marrow of the new mutant, which in principle should resemble the Cd79b knockout mouse. Indeed there were several features in common between the new mutant and the Cd79b knockout, but also some striking differences. As in the original report on the KO mouse, we found that the B cell development seemed to stop at the CD43posB220pos stage. However, in contrast to the previous work, we found dramatic changes in the distribution of the cells in fractions A-C. Using CD24 and BP-1 surface staining13, we found that fraction C′ was virtually absent in the CD79b mutant mouse, whereas fractions B and C showed a relative accumulation (Fig. 2b). Such accumulation would be expected if the mutant B cells were unable to receive a signal via the BCR, as both fractions are immediate precursors of the C′ cells14. This result suggests that the Cd79b gene product is necessary for progression into the C′ prime stage, and fits well with current knowledge of the B cell development process, which holds that cells in fraction C′ are large, proliferating pre-B cells that have recently received a functional signal via the pre-BCR complex15.
Figure 2. Phenotype of the bone marrow cells from Cd79b mutant and wild mice for pro-B to pre-B transition markers.
a) Surface expression of B220 and CD43 on bone marrow cells of mutant and wild type mouse.
b) A, B, C, C′ fractions in bone marrow cells of mutant and wild type mouse defined by surface expression of BP-1 and CD24.
c) Percentages (mean, standard deviation) of A, B, C, C′ fractions of in bone marrow cells of mutant and wild type (n=4 in each group). Control mice consisted of 2 wild-type and 2 heterozygous animals.
FACS staining: All procedures were performed as described in Figure 1. Following fluorochrome-conjugated antibodies were used for staining: anti-B220-APC-Alexa750 (clone RA3–6B2, eBioscience, San Diego, CA, USA), anti-CD43-APC (BioLegend, San Diego, CA, USA, clone 1B11), anti-CD24-FITC (BD Pharmigen, San Jose, CA, USA, clone M1/69), anti-BP1-PE (BD, clone BP-1). Bone marrow from 2–4 months old female mice was used for staining.
There are two potential reasons why we obtained different results from those previously published on the Cd79b KO mouse. The first is that C and C′ populations in B6 mice have been difficult to discriminate by FACS (personal communication from R. Hardy). The last decade has seen a great deal of technological improvement in flow-cytometry that allowed us to overcome this problem. The second reason is related to the methods by which the mutations were generated. Our mutant mouse is a spontaneous mutant that has only a single point mutation, the remainder of its genome coming from the inbred line. This provides a unique opportunity to estimate precisely where the block in development occurs, without the effect of any other potential confounding factors that may exist in a knock-out mouse, where it is difficult to rule out the effect of other genes that may not have been removed during the backcrossing process.
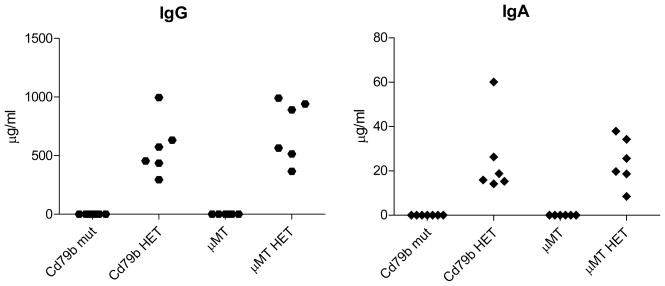
Some investigators have found low levels of IgG, IgA in μMT B cell knockout (BcKO) mice16, which have a block in the development of B cells at a stage similar to our mutant. Others, however, have not17, and human patients with similar mutations lack IgA18. We therefore measured levels of total IgA and IgG in the blood of Cd79b mutant, in control heterozygous mice, and in μMT BcKO mice and their corresponding controls. We found neither IgA nor IgG in the Cd79b mutant or the μMT BcKO mice (Figure 3). Although it is still not clear why our results in the μMT mice differ from those of Macpherson et al, one hypothesis is that it relates to differences in the flora of the different mouse colonies. In addition, Hasan et al have shown that there is additional locus not linked to immunoglobulin locus which is critical for differences in immunoglobulin production between μMT BcKO mice on BALB/c and B6 backgrounds. The result regarding the absence of immunoglobulins in Cd79b mutant is quite straightforward and is in agreement with aglobulinemias in Cd79b mutant patients4; 5.
Figure 3. Serum levels of IgG and IgA in CD79b mutant (mut), μMT mice and their corresponding heterozygous (HET) littermates.
Immunoglobulin levels were measured in serum samples using ELISA kits from Immunology Consultants Lab Inc (Newberg, OR, USA). Mice were females, 3–6 months of age, the background strain for all mice was B10.A. The original mutant and wild-type mice were obtained from the NIAID contract facility at Taconic Farms, and bred to N2 generation to obtain mutant and heterozygous littermates.
Different mutations in the Cd79b gene can lead to different outcomes. For example, leukemia has been reported in human patients with mutations in Cd79b gene that lead to changes in the protein but not to its complete or significant ablation19. Notably, the mutation found in the new mouse reported here is remarkably similar to the mutations found in two immunodeficient patients that had mutations in the Cd79b gene. Indeed, both patients had mutations leading to a stop codon in the 3rd exon of the Cd79b gene, and had no or extremely low numbers of B cells and antibodies4; 5. These results suggest that this new mutant strain could be a useful tool for immunological research into the human immunodeficiencies, as it closely resembles the phenotype of the immunodeficient human patients; it has a homogeneous inbred genome, a congenic marker, and a wild type strain that differs only by this single mutation.
Acknowledgments
This research was supported by the Intramural Research Program of the NIAID, NIH. We thank: BJ Fowlkes and Xianyu Zhang for suggestions about staining of bone marrow samples, and for sharing antibodies; Andy Johnson for suggestions regarding gene mapping; Debbie Wagner for help in typing mice; LCMI/NIAID personnel, Stefan Muljo, and Richard Hardy for comments and discussions; Alison Brown and Oleg Iartchouk from Partners HealthCare Center for Personalized Genetic Medicine for excellent service.
Footnotes
Conflict of interest
None.
References
- 1.Kumar A, Teuber SS, Gershwin ME. Current perspectives on primary immunodeficiency diseases. Clin Dev Immunol. 2006;13(2–4):223–259. doi: 10.1080/17402520600800705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361(6409):226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72(2):279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 4.Dobbs AK, Yang T, Farmer D, Kager L, Parolini O, Conley ME. Cutting edge: a hypomorphic mutation in Igbeta (CD79b) in a patient with immunodeficiency and a leaky defect in B cell development. J Immunol. 2007;179(4):2055–2059. doi: 10.4049/jimmunol.179.4.2055. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari S, Lougaris V, Caraffi S, Zuntini R, Yang J, Soresina A, et al. Mutations of the Igbeta gene cause agammaglobulinemia in man. J Exp Med. 2007;204(9):2047–2051. doi: 10.1084/jem.20070264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science. 1999;286(5446):1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 7.Alpan O, Rudomen G, Matzinger P. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J Immunol. 2001;166(8):4843–4852. doi: 10.4049/jimmunol.166.8.4843. [DOI] [PubMed] [Google Scholar]
- 8.Hermanson GG, Eisenberg D, Kincade PW, Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on beta-lineage cells. Proc Natl Acad Sci U S A. 1988;85(18):6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- 10.Campbell KS, Hager EJ, Friedrich RJ, Cambier JC. IgM antigen receptor complex contains phosphoprotein products of B29 and mb-1 genes. Proc Natl Acad Sci U S A. 1991;88(9):3982–3986. doi: 10.1073/pnas.88.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig beta. Science. 1996;272(5260):411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 12.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5(6):527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 14.Ehlich A, Martin V, Muller W, Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994;4(7):573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 15.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26(6):703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Macpherson AJ, Lamarre A, McCoy K, Harriman GR, Odermatt B, Dougan G, et al. IgA production without mu or delta chain expression in developing B cells. Nat Immunol. 2001;2(7):625–631. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 17.Hasan M, Polic B, Bralic M, Jonjic S, Rajewsky K. Incomplete block of B cell development and immunoglobulin production in mice carrying the muMT mutation on the BALB/c background. Eur J Immunol. 2002;32(12):3463–3471. doi: 10.1002/1521-4141(200212)32:12<3463::AID-IMMU3463>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Pan Q, Matamoros N, Bjorkander J, Conley ME, Hammarstrom L. Lack of IgA in C(mu)-deficient patients. Nat Immunol. 2002;3(7):595. doi: 10.1038/ni0702-595. author reply 596. [DOI] [PubMed] [Google Scholar]
- 19.Gordon MS, Kato RM, Lansigan F, Thompson AA, Wall R, Rawlings DJ. Aberrant B cell receptor signaling from B29 (Igbeta, CD79b) gene mutations of chronic lymphocytic leukemia B cells. Proc Natl Acad Sci U S A. 2000;97(10):5504–5509. doi: 10.1073/pnas.090087097. [DOI] [PMC free article] [PubMed] [Google Scholar]