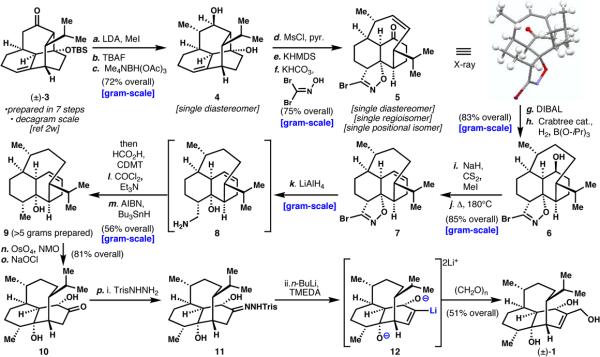
Scheme 1. Total synthesis of vinigrol (1).
Reagents and conditions: (a) LDA (1.3 equiv.), MeI (1.6 equiv.), THF, −78 to 0 °C, 3.3 h; (b) TBAF (3.2 equiv.), THF, 50 °C, 3 h; (c) Me4NBH(OAc)3 (4.0 equiv.), AcOH:MeCN:THF = 1:1:1, 23 °C, 1.5 h, 72% over 3 steps; (d) MsCl (1.2 equiv.), Pyridine, 0 °C, 2.5 h; (e) KHMDS (1.1 equiv.), THF, 0 to 23 °C, 35 min, 85% over 2 steps; (f) KHCO3 (3.0 equiv.), Br2 C=NOH (1.5 equiv.), EtOAc, 23 °C, 45 min, 88%; (g) DIBAL (1.2 equiv.), DCM, −78 °C, 1 h, 95%; (h) Crabtree's catalyst (0.2 equiv.), B(O-iPr)3 (1.0 equiv.), H2 (1 atm), DCE, 80 °C, 8 h, 87%; (i) NaH (10 equiv.), CS2 (20 equiv.), MeI (40 equiv.), THF, 0 to 23 °C, 15 h, 88%; (j) o-DCB, 180 °C, 3 h, 96%; (k) LiAlH4 (20 equiv.), THF, 0 to 23 °C, 12 h; HCOOH (2.0 equiv.), CDMT (2.1 equiv.), NMM (2.2 equiv.), DMAP (0.1 equiv.), DCM, 23 °C, 1 h, 81%; (l) COCl2 (1.0 equiv.), Et3N (15 equiv.), DCM, −20 °C, 20 min, 76%; (m) AIBN (3.0 equiv.), Bu3SnH (9.8 equiv.), Toluene, 100 °C, 2.5 h, 91%; (n) OsO4 (0.1 equiv.), NMO (1.3 equiv.), Acetone:H2O = 3:1, 23 °C, 12 h, 95%; (o) NaOCl (1.5 equiv.), TEMPO (0.1 equiv.), KBr (0.1 equiv.), a.q 5% NaHCO3:DCM = 2:5, 0 °C, 1.5 h, 85%; (p) TrisNHNH2 (2.0 equiv.), DCM, 23 °C, 5 h; n-BuLi (4.0 equiv.), (CH2O)n (30 equiv.), TMEDA:THF = 2:1, −78 to 23 °C, 3 h, 51% overall.