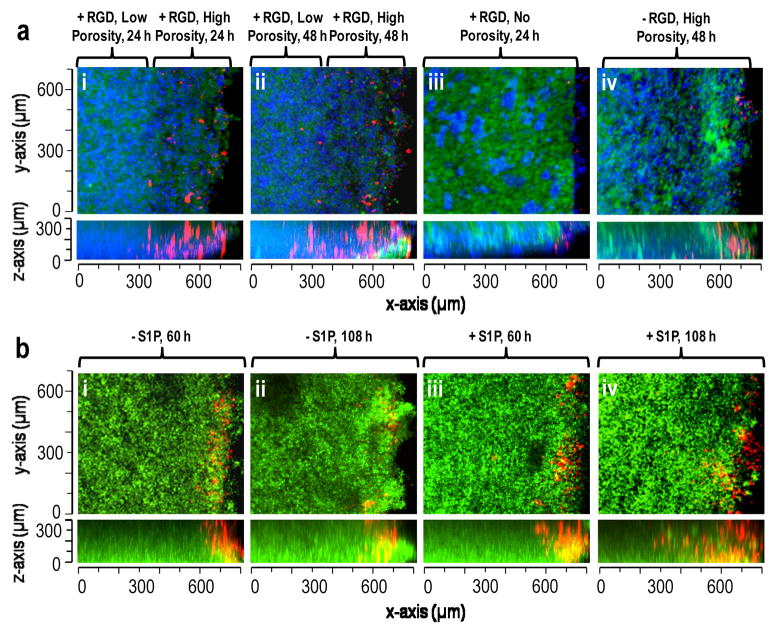
Figure 6.
S1P, porosity and RGD peptide affected endothelial cell migration in modular scaffolds. (a) Migration of Vybrant DiI stained endothelial cells (red) into scaffolds composed of PEG8-VS/PEG8-amine microspheres (green), PEG8-acrylate/PEG8-amine microspheres and PEG8-VS/BSA microspheres (blue). Scaffolds were formed with: (i) RGD peptide and a highly porous 250–300 μm upper layer, imaged 24 h after cell seeding, (ii) Same location within scaffold from (i) at 48 h, (iii) RGD peptide but no porogenic microspheres, 24 h, (iv) No RGD peptide but with porogenic microspheres, 48 h. (b) Migration of endothelial cells (red) into scaffolds with uniform porosity composed of PEG8-VS/PEG8-amine microspheres, PEG8-acrylate/PEG8-amine microspheres and PEG8-VS/BSA microspheres (with or without S1P-loaded, green): (i) 60 h after cell seeding without S1P-loading, (ii) 108 h without S1P-loading, (iii) 60 h with S1P-loading, (iv) 108 h with S1P-loading. The maximum extent of migration of endothelial cells was recorded at 50 μm increments within each scaffold. The average rate of migration increased from 2.57±0.76 μm/h to 5.4±1.02 μm/h when S1P was released from the PEG8-VS/BSA microspheres.