Abstract
There is considerable interest in developing an 18F-labeled PET myocardial perfusion agent. Rhodamine dyes share several properties with 99mTc-MIBI, the most commonly used single-photon myocardial perfusion agent, suggesting that an 18F-labeled rhodamine dye might prove useful for this application. In addition to being lipophilic cations, like 99mTc-MIBI, rhodamine dyes are known to accumulate in the myocardium and are substrates for Pgp, the protein implicated in MDR1 multidrug resistance. As the first step in determining whether 18F-labeled rhodamines might be useful as myocardial perfusion agents for PET, our objective was to develop synthetic methods for preparing the 18F-labeled compounds so that they could be evaluated in vivo. Rhodamine B was chosen as the prototype compound for development of the synthesis because the ethyl substituents on the amine moieties of rhodamine B protect them from side reactions, thus eliminating the need to include (and subsequently remove) protecting groups. The 2′-[18F]fluoroethyl ester of rhodamine B was synthesized by heating rhodamine B lactone with [18F]fluoroethyltosylate in acetonitrile at 165°C for 30 min.using [18F]fluoroethyl tosylate, which was prepared by the reaction of ethyleneglycol ditosylate with Kryptofix 2.2.2, K2CO3, and [18F]NaF in acetonitrile for 10 min. at 90°C. The product was purified by semi-preparative HPLC to produce the 2′-[18F]-fluoroethylester in >97% radiochemical purity with a specific activity of 1.3 GBq/μmol, an isolated decay corrected yield of 35%, and a total synthesis time of 90 min.
Keywords: fluorine-18, positron emission tomography, rhodamine B, myocardial perfusion imaging
1. Introduction
There is considerable interest in developing an 18F-labeled compound for PET myocardial perfusion imaging. This interest arises primarily because of the limitations of other PET radionuclides currently used for this application. These limitations include the high cost of 82Rb (from the 82Sr/82Rb generator) and the limited availability of [13N]NH3 (because of the short half-life of 13N). There is some interest in Cu radionuclides for this application (Packard et al., 2002, Wallhaus et al., 2001), primarily with 64Cu because its half-life (12.7 h) is long enough to allow shipping from central production sites, but the positron yield of 18F is greater than that of 64Cu (97% vs. 18%), the shorter half-life of 18F (110 min.) allows repeated studies within the same day, and the distribution networks that have been established for [18F]FDG have demonstrated that production of 18F-labeled radiopharmaceuticals at central sites is a reasonable alternative to on-site production. The 18F compounds that have been reported to date as possible myocardial perfusion agents include quaternary ammonium salts (Studenov et al., 2001), triphenylphosphonium compounds (Ravert et al., 2004, Madar et al., 2006, Madar et al., 2007b, Shoup et al., 2005, Madar et al., 2007a), rotenone (Marshall et al., 2004), and BMS-747158-02, a modified version of the pyridazinone insecticide pyridaben designed to target mitochondrial complex I (Huisman et al., 2008).
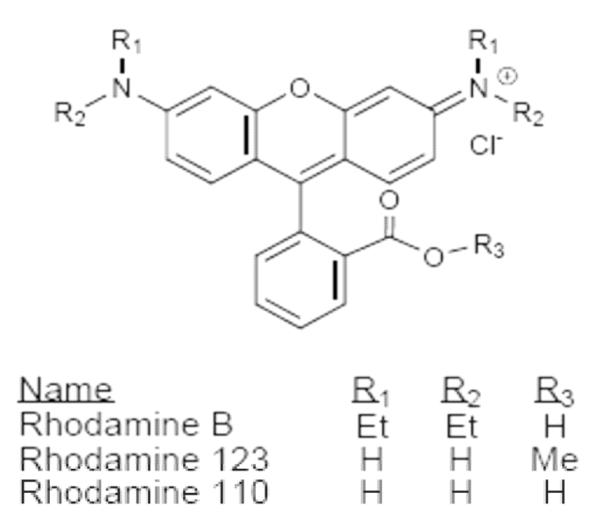
There are several reasons that 18F-labeled rhodamines (Fig. 1) are of interest as possible PET myocardial perfusion agents. First of all, as is the case for the single-photon myocardial perfusion agents 99mTc-MIBI and 99mTc-tetrofosmin and many of the 18F-labeled compounds listed above, they are lipophilic cations. Other properties that non-radiolabeled rhodamines have in common with 99mTc-MIBI include accumulation in mitochondria in proportion to mitochondrial membrane potential (Lacerda et al., 2005, Reungpatthanaphong et al., 2003, Hu et al., 2000, Lampidis et al., 1985), accumulation in brain tumors (Powers et al., 1988, Packard et al., 1997), and being substrates for Pgp, a protein implicated in multidrug resistance (Rusiecka et al., 2008, De Moerloose et al., 1999). Perhaps most significantly, Vora and Dhalla showed that non-radiolabeled rhodamine 123 accumulated in the rat heart (Vora et al., 1992). Additionally, Kassis and co-workers have investigated the potential use of radioiodinated rhodamine 123 in tumor diagnosis or therapy (Kinsey et al., 1987, Kinsey et al., 1989, Harapanhalli et al., 1998).
Fig. 1.
Examples of rhodamine dyes
There are no previous reports of the synthesis of an 18F-labeled rhodamine, so the first step in this investigation was the development of a method by which rhodamines could be labeled with 18F. Rhodamine B was chosen as the prototype compound in this series primarily because the ethyl substituents on the amines of the xanthene ring system preclude the need to protect them from possible side reactions. An additional consideration is that rhodamine B is itself a substrate for Pgp (Rusiecka et al., 2008) and a marker of mitochondrial membrane potential (Reungpatthanaphong et al., 2003).
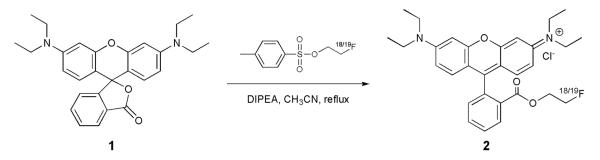
The general synthetic approach used was to prepare the 2′-[18F]fluoroethyl ester of the benzoic acid moiety of the parent compound, a method analogous to the route used by Yoon, et al. for the preparation of 18F-labeled flumazenil (Yoon et al., 2003). In the present work, we describe the one-pot synthesis of 2′-[18F]fluoroethylrhodamine B (1) from rhodamine B lactone (2) using [18F]fluoroethyl tosylate ([18F]FETos) as the fluorination reagent as well as the synthesis and characterization of the non-radiolabeled analog. A preliminary account of this work was presented in abstract form (Heinrich et al., 2007, Heinrich et al., 2008).
2. Experimental
2.1. General
Rhodamine B lactone (>97%) was purchased from MP Biomedicals (Solon, OH). Ethylene glycol ditosylate (>97%) was purchased from Sigma-Aldrich (St. Louis, MO). Extra dry reagent grade acetonitrile and Kryptofix® (K2.2.2) (98%) were purchased from Fluka (St. Louis, MO). Potasssium carbonate (99.997%) was purchased from Alfa Aesar (Ward Hill, MA). Other solvents and reagents were of the highest grade commercially available and were used as received unless otherwise noted. Thin-layer chromatography (TLC) was performed using Silicagel IB-F coated plastic sheets from J.T. Baker (Phillipsburg, NJ). 1H-Nuclear magnetic resonance (NMR) spectra were obtained using a Varian 400 spectrometer (Palo Alto, CA). Chemical shifts are reported as δ values. Coupling constants are reported in hertz. Multiplicity is defined by s (singlet), d (doublet), t (triplet), q (quadruplet) and m (multiplet). Mass spectra were obtained through the courtesy of Prof. Elena V. Rybak-Akimova (Tufts University) using a Thermo- Finnigan LTQ Mass Spectrometer is ESI-MS mode. Fluorine-18 (in water) was purchased from Cardinal Healthcare (Woburn, MA) or obtained as a gift from Massachusetts General Hospital.
2.2 Purification and quality control
Analytical high-performance liquid chromatography (HPLC) was carried out using an HITACHI 7000 system including an L-7455 diode array detector, an L-7100 pump, and a D-7000 interface. The radiometric HPLC detector was comprised of Canberra nuclear instrumentation modules and optimized for 511 keV photons. A Hitachi LaChrom PuroSphere Star C18e column (4 × 30 mm; 3 μm) was used for analytical measurements. The solvent system was 0.1% trifluoroacetic acid (TFA) in water (solvent A) and 0.1% TFA in acetonitrile (solvent B) at a flow rate of 1 mL/min at room temperature. The solvent gradient was 0-15 min (30%-70% B), 15-25 min. (70% B).
For semi-preparative HPLC, an ISCO system comprised of an ISCO V4 variable wavelength uv-visible detector (operated at λ=550 nm), an ISCO 2300 HPLC pump, a Canberra gamma detector, and a Grace Apollo C18 column (10 × 250 mm; 5μm) was used. Preparative HPLC method (isocratic): 40% 0.1% trifluoroacetic acid (TFA) in water (solvent A); 60% 0.1% TFA in acetonitrile; flow rate — 5 mL/min.; room temperature.
Radiofluorination yields were determined by thin-layer chromatography using silica gel plates and chloroform:methanol (8:1 v/v) as the solvent. After they were developed, the TLC strips were cut into 1 cm pieces and counted with a Packard Cobra gamma counter.
2.3. Animal studies
Animal studies were carried out under a protocol approved by the Children’s Hospital Boston Institutional Animal Care and Use Committee. Imaging studies were carried out using a Siemens Focus 120 microPET system. The HPLC-purified compound was evaporated to dryness (to remove acetonitrile and trifluoractic acid) and redissolved in a solution of 10% ethanol in saline for injection. Animals were injected with 3.7-7.4 MBq (100-200 μCi) of 2′-[18F]fluorethylrhodamine B in 100 μL of 10% ethanol in saline. Immediately after injection, the animals were anesthetized with isofluorane (2-4% in air) and transferred to the microPET system. Image acquisition was initiated approximately 5 min. after injection. The total imaging time was 1 h.
2.4. Synthesis of non-radioactive 2′-fluoroethylrhodamine B
Synthesis of 2-fluoroethyl tosylate (FETos)
FETos was prepared from 2-fluoroethanol and p-toluene sulfonyl chloride in dry pyridine under nitrogen at 0°C for 4.5 h as described by Parenty, et al. (Parenty et al., 2005). The product was obtained as an oil, which solidified after several days in the refrigerator.
Synthesis of 2′-fluoroethyl rhodamine B (FERhB)
The nonradioactive 2′-fluoroethyl ester of rhodamine B (2) was prepared by transesterification of rhodamine B lactone (1) with FETos (Scheme 1). 250 mg of 1 (0.56 mmol) were dissolved in 10 mL acetonitrile to which 146 mg FETos (0.67 mmol, previously dissolved in 2 mL acetonitrile) and 0.5 mL (2.94 mmol) diisopropylethylamine (DIPEA) were added. The solution was refluxed and the reaction was monitored by HPLC. After 24 h, 97% of the rhodamine B lactone starting material had been converted to the 2′-fluoroethyl ester, at which time the heating was discontinued, the reaction mixture was allowed to cool to room temperature, and then evaporated to dryness to give approx. 400 mg of crude product as a dark red oil. 10 mg of the crude product was purified by semi-preparative HPLC to provide 9.5 mg (95%) of purified FERhB as a purple oil.
Scheme 1.
Synthesis of 2′-fluoroethyl rhodamine B.
Compound 1
TLC: Rf = 0.5 (chloroform/methanol, 8:1). 1H-NMR (CDCl3, 600 MHz): δ=1.33 (t, 3J=7.1 Hz, 12H, 4CH2CH3), 3.62 (q, 3J=7.1 Hz, 8H, 4CH2CH3), 4.21–4.24 (m, 1H, CH2CH2 ), 4.25–4.27 (m, 1H, CH2CH2 ), 4.34–4.36 (m, 1H, CH2CH2 ), 4.44–4.46 (m, 1H, CH2CH2 ), 6.82-6.86(m, 4HAr), 7.05–7.09 (m, 2HAr), 7.34 (d, 3J=7.2 Hz, 1HAr), 7.75 (dd, 3J=7.1Hz, 1HAr), 7.82 (dd, 3J=7.1Hz, 1HAr), 8.34 (d, 3J=7.2 Hz, 1HAr). Mass spec: calculated 489.25 Found 489.38
2.5. Radiolabeling
Drying procedure for [18F]fluoride
The aqueous [18F]fluoride solution (100 μL to 1 mL, 75 MBq to 750 MBq 18F) was added to a solution of Kryptofix®2.2.2. (5 mg, 13.3 μmol) and potassium carbonate (0.9 mg, 6.7 μmol) in 0.5 mL acetonitrile-water (9:1 v/v) and dried azeotropically by addition of 10 mL CH3CN (3 × 3.3 mL) under a stream of nitrogen at 90°C. A final azeotropic drying was performed with 3 × 0.5 mL of extra dry CH3CN.
Synthesis of [18F]FETos
Ethyleneglycol-1,2-distosylate (2.5 mg in 0.5 mL extra dry acetonitrile) was added to the dried Kryptofix®2.2.2./K2CO3[18F]fluoride mixture and heated at 100°C for 10 min. in a sealed Pierce “V-vial” to produce the [18F]FETos in 80-90% yield. The product may be purified by diluting the reaction mixture with 4 mL diethyl ether and passing through a Sep-Pak® silica gel cartridge. Kryptofix®, K2CO3, and excess [18F]fluoride are retained on the cartridge, and the product is eluted with 5 mL diethyl ether, which is then evaporated at 50°C under a steam of nitrogen. Excess ethyleneglycol-1,2- distosylate is not separated from [18F]FETos using this procedure. The procedure requires approximately 25 min. and provides [18F]FETos in >95% radiochemical purity with an overall radiochemical yield of 30 to 50%.
Preparation of [18F]FERhB (1)
5 mg rhodamine B lactone dissolved in 0.8 mL anhydrous acetonitrile and 20 μL of DIPEA were added sequentially to the unpurified [18F]FETos (above) in the original reaction vial, the vial was sealed, the septum in the cap was fitted with a ventilation needle (30 g), and reaction mixture was heated for 30 min. at 165°C. The final product, [18F]FERhB, was obtained in an overall radiochemical yield of 35% after purification by solid-phase extraction (silica gel Sep-Pak® cartridge (Waters), CHCl3:methanol, 4:1) or semi-preparative HPLC (tR=13.2 min). The total reaction time using this method, including the drying of the [18F]fluoride and preparative HPLC, is approximately 90 min. The identity of the product was confirmed by analytical HPLC and TLC using non-radioactive compound 2′-fluoroethyl rhodamine B as a reference. On analytical HPLC, the retention time of [18F]FERhB is 9.6 min., and the Rf (TLC) is 0.5 (chloroform/methanol 8:1).
3. Results and Discussion
3.1. Synthesis of the non-radioactive compound
An authentic sample of 2′-fluoroethyl ester of rhodamine B (FERhB, 2) was prepared and chemically characterized for use as a reference compound for the HPLC characterization of the 18F-labeled compound. The synthetic route is outlined in Scheme 1. The nonradioactive fluoroethyl tosylate (FETos) was synthesized according to literature (Parenty et al., 2005), and the non-radioactive 2′-fluorethyl ester of rhodamine B was prepared by fluoroethylation of rhodamine B lactone (1) with FETos in the presence of DIPEA. The conversion from the lactone to the 2′-fluoroethylester was monitored by HPLC and was determined to be 97% after 24 h under reflux. The product was purified by semi-preparative HPLC, isolated as a purple oil in 95% yield, and characterized by NMR and mass spectroscopy.
3.2. Synthesis of 18F-labeled rhodamine B
The radiosynthesis of [18F]FERhB was accomplished using a one-pot synthesis in which the aqueous [18F]fluoride solution was evaporated in a Pierce “V” vial after addition of Kryptofix® and K2CO3, ethylene glycol ditosylate dissolved in acetonitrile was added to the dried residue, and the solution was heated for 10 min. to give [18F]fluoroethyltosylate. This reaction mixture was cooled to room temperature, and a solution of rhodamine B lactone in acetonitrile and DIPEA were added to the unpurified precursor. This solution was then heated for 30 min. to give the 18F-labeled rhodamine. The one-pot synthesis was developed after it was observed that a large amount (>50%) of the [18F]FETos precursor was lost during evaporation of the diethyl ether solvent when solid-phase extraction was used to purify this material. While optimizing the reaction conditions, we observed that the yield of the reaction was a much higher if the solvent was allowed to slowly evaporate during the reaction, so a small gauge needle was inserted in the reaction vial in all subsequent studies. Possible reasons for this include the increased concentration of the precursors as the solvent evaporates and opening of the lactone ring on heating. These optimized conditions produced the final product in a decay corrected yield of 35%, 97% radiochemical purity, specific activity - 1.3 GBq/μmol, and a total synthesis time of approximately 90 min.
3.3. Small-animal PET imaging
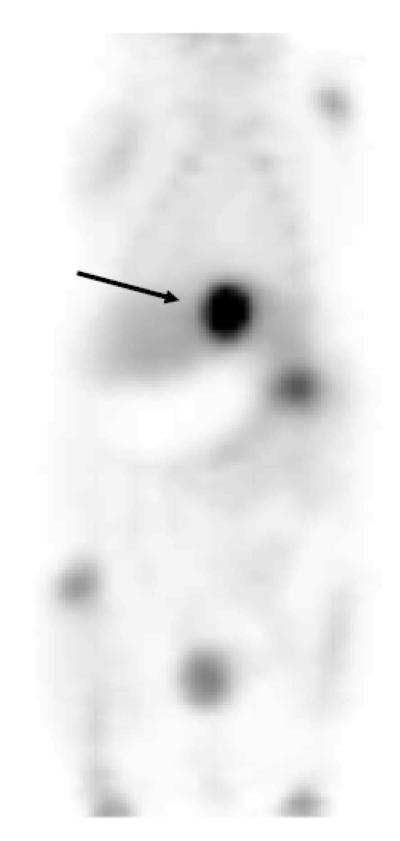
The biodistribution of 2′-[18F]fluorethylrhodamine B was examined in mice using microPET. An example of one of these studies is shown in Figure 2. The microPET study reveals that there is no appreciable uptake in the myocardium. Small, but noticeable, uptake of 18F was observed in the ribs and long bones. This is presumably due to the presence of a small amount of free 18F fluoride in the [18F]FERhB preparation (<3% by TLC). There is significant uptake in the gall bladder, and subsequently, the intestine. This observation is consistent with the results obtained by Harapanhalli, et al., who also observed excretion into the small intestine with minimal accumulation in the heart (Harapanhalli et al., 1998). We suspect that this may be due to hydrolysis of the ester in vivo. To evaluate this possibility, a sample of bile was collected from a mouse sacrificed 1 h post-injection and assayed using the TLC system described above the assay the HPLC purified product. This analysis revealed that less than 10% of the 18F activity in the bile was present as [18F]FERhB and less than 20% was free 18F-fluoride. The remainder of the activity (>70%) was present as a new peak (Rf=0.3-0.4), presumably 2-[18F]fluoroethanol and/or one or more of its metabolites. Additional studies are currently underway to more completely characterize this material. More importantly, we are evaluating alternative labeling strategies that may be less susceptible to in vivo degradation.
Fig. 2.
MicroPET image (1 h) of a mouse injected with [18F]2′-fluoroethylrhodamine B. Note high concentration of radioactivity in the gall bladder (arrow).
4. Conclusions
Using rhodamine B as the prototype compound, we have developed a synthetic method for radiolabeling rhodamine dyes with 18F as a first step in evaluating these compounds as possible PET tracers for myocardial perfusion imaging. Fluorination was accomplished by synthesis of the 2′-fluoroethyl ester using rhodamine B lactone as the starting material and 2-tosylethylfluoride as the prosthetic group. Both the non-radioactive and 18F-labeled compounds were prepared using the same general method. The 18F-labeled compound was obtained in reasonably high radiochemical yield (35%) and high radiochemical purity (>97%). The specific activity is 1.3 GBq/μmol, and the total synthesis time is approximately 90 min. Preliminary microPET studies of the biodistribution of the 18F-labeled compound in mice, however, showed that rather than accumulating in the heart, the compound was rapidly excreted through the gall bladder and into the intestines. TLC analysis of the bile revealed that activity in the bile was not primarily either intact [18F]FERhB or 18F-fluoride suggesting in vivo hydrolysis of the ester to produce 2′-[18F]fluoroethanol and its metabolites. Additional studies are currently underway to more completely characterize the route of decomposition and to determine if this may be the reason for this unexpected result thereby providing a basis for deciding if these compounds merit further examination as potential myocardial perfusion agents.
Acknowledgements
We gratefully acknowledge the assistance of the cyclotron facility at Massachusetts General Hospital, especially John A. Correia, Ph.D., the Brigham and Woman’s Hospital Radiopharmacy, especially Patrick W. Gallagher, Ph.D. and Cardinal Health Inc. who provided the 18F necessary to carry out these experiments. The mass spectrometer used in this investigation was purchased with the support of NSF grant #MRI 0320783 to Tufts University. This investigation was supported by NIH grant 5 R01 CA94338 (ABP) and the Children’s Hospital Boston Radiology Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- De Moerloose B, Van de Wiele C, Dhooge C, Philippe J, Speleman F, Benoit Y, Laureys G, Dierckx RA. Technetium-99m sestamibi imaging in paediatric neuroblastoma and ganglioneuroma and its relation to P-glycoprotein. Eur. J. Nucl. Med. 1999;26:396–403. doi: 10.1007/s002590050403. [DOI] [PubMed] [Google Scholar]
- Harapanhalli RS, Roy AM, Adelstein SJ, Kassis AI. [125I/127I/131I]Iodorhodamine: Synthesis, cellular localization, and biodistribution in athymic mice bearing human tumor xenografts and comparison with [99mTc]hexakis(2-methoxyisobutylisonitrile) J. Med. Chem. 1998;41:2111–2117. doi: 10.1021/jm970691i. [DOI] [PubMed] [Google Scholar]
- Heinrich TK, Fahey F, Dunning P, Snay E, Treves ST, Packard AB. Synthesis and initial in vivo characterization of 18F-labeled rhodamine B: A potential PET myocardial perfusion agent. Society of Nuclear Medicine, 55th Annual Meeting; New Orleans, LA. 2008. p. 302P. [Google Scholar]
- Heinrich TK, Treves ST, Packard AB. Development of 18F-labeled rhodamine B derivatives for myocardial perfusion imaging with PET. AMI/SMI Joint Molecular Imaging Conference; Providence, RI. 2007. [Google Scholar]
- Hu Y, Moraes CT, Savaraj N, Priebe W, Lampidis TJ. Rho(0) tumor cells: a model for studying whether mitochondria are targets for rhodamine 123, doxorubicin, and other drugs. Biochem. Pharmacol. 2000;60:1897–1905. doi: 10.1016/s0006-2952(00)00513-x. [DOI] [PubMed] [Google Scholar]
- Huisman MC, Higuchi T, Reder S, Nekolla SG, Poethko T, Wester H-J, Ziegler SI, Casebier DS, Robinson SP, Schwaiger M. Initial Characterization of an 18F-Labeled Myocardial Perfusion Tracer. J. Nucl. Med. 2008;49:630–636. doi: 10.2967/jnumed.107.044727. [DOI] [PubMed] [Google Scholar]
- Kinsey BM, Kassis AI, Fayad F, Layne WW, Adelstein SJ. Synthesis and biological studies of iodinated (127/125I) derivatives of rhodamine 123. J. Med. Chem. 1987;30:1757–1761. doi: 10.1021/jm00393a013. [DOI] [PubMed] [Google Scholar]
- Kinsey BM, Van den Abbeele AD, Adelstein SJ, Kassis AI. Absence of preferential uptake of [125I]iododihydrorhodamine 123 by four human tumor xenografts. Cancer Res. 1989;49:5986–5988. [PubMed] [Google Scholar]
- Lacerda SH, Abraham B, Stringfellow TC, Indig GL. Photophysical, photochemical, and tumor-selectivity properties of bromine derivatives of rhodamine-123. Photochem. Photobiol. 2005;81:1430–1438. doi: 10.1562/2005-08-05-RA-639. [DOI] [PubMed] [Google Scholar]
- Lampidis TJ, Hasin Y, Weiss MJ, Chen LB. Selective killing of carcinoma cells “in vitro” by lipophilic-cationic compounds: a cellular basis. Biomed. Pharmacother. 1985;39:220–226. [PubMed] [Google Scholar]
- Madar I, Gao D, Ravert H, Chen L, Pomper M, Dannals R, Wei C. 18F-Fluorobenzyltriphenyl phosphonium PET detects area-specific apoptosis in the aging myocardium. Society of Nuclear Medicine, 54th Annual Meeting; Washington, DC. 2007a. p. 166P. [Google Scholar]
- Madar I, Ravert H, DiPaula A, Du Y, Dannals RF, Becker L. Assessment of severity of coronary artery stenosis in a canine model using the PET agent 18F-fluorobenzyl triphenyl phosphonium: Comparison with 99mTc-Tetrofosmin. J. Nucl. Med. 2007b;48:1021–1030. doi: 10.2967/jnumed.106.038778. [DOI] [PubMed] [Google Scholar]
- Madar I, Ravert HT, Du Y, Hilton J, Volokh L, Dannals RF, Frost JJ, Hare JM. Characterization of uptake of the new PET imaging compound 18F-fluorobenzyl triphenyl phosphonium in dog myocardium. J. Nucl. Med. 2006;47:1359–1366. [PubMed] [Google Scholar]
- Marshall RC, Powers-Risius P, Reutter BW, O’Neil JP, La Belle M, Huesman RH, VanBrocklin HF. Kinetic analysis of 18F-fluorodihydrorotenone as a deposited myocardial flow tracer: Comparison to 201Tl. J. Nucl. Med. 2004;45:1950–1959. [PubMed] [Google Scholar]
- Packard AB, Barbarics E, Kronauge JF, Wen PY, Day PJ, Jones AG. Comparison of uptake of 99mTc-alkylisonitriles in the rat 9L gliosarcoma tumor model. Nucl. Med. Biol. 1997;24:21–25. doi: 10.1016/s0969-8051(96)00152-7. [DOI] [PubMed] [Google Scholar]
- Packard AB, Kronauge JF, Barbarics E, Kiani S, Treves ST. Synthesis and biodistribution of a Lipophilic 64Cu-labeled monocationic Copper(II) complex. Nuc. Med. Biol. 2002;29:289–294. doi: 10.1016/s0969-8051(02)00285-8. [DOI] [PubMed] [Google Scholar]
- Parenty ADC, Smith LV, Cronin L. An unusual substitution reaction directed by an intramolecular re-arrangement. Tetrahedron. 2005;61:8410–8418. [Google Scholar]
- Powers SK, Ellington K. Selective retention of rhodamine-123 by malignant glioma in the rat. J. Neurooncol. 1988;6:343–347. doi: 10.1007/BF00177430. [DOI] [PubMed] [Google Scholar]
- Ravert HT, Madar I, Dannals RF. Radiosynthesis of 3-[18F]fluoropropyl and 4-[18F]fluorobenzyl triarylphosphonium ions. J. Label. Compd. Radiopharm. 2004;47:469–476. [Google Scholar]
- Reungpatthanaphong P, Dechsupa S, Meesungnoen J, Loetchutinat C, Mankhetkorn S. Rhodamine B as a mitochondrial probe for measurement and monitoring of mitochondrial membrane potential in drug-sensitive and -resistant cells. J. Biochem. Biophys. Methods. 2003;57:1–16. doi: 10.1016/s0165-022x(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Rusiecka I, Skladanowski AC. Induction of the multixenobiotic/multidrug resistance system in various cell lines in response to perfluorinated carboxylic acids. Acta Biochim. Pol. 2008;55:329–337. [PubMed] [Google Scholar]
- Shoup TM, Elmaleh DR, Brownell A-L, Pitman JT, Zhu A, Wang X, Correia JA, Fischman AJ. Evaluation of (4-[18F]fluorophenyl)triphenylphosphonium ion as a potential myocardial blood flow agent for PET. 52nd Annual Meeting of the Society of Nuclear Medicine; Toronto, ON. 2005. [Google Scholar]
- Studenov AR, Berridge MS. Synthesis and properties of 18F-labeled potential myocardial blood flow tracers. Nuc. Med. Biol. 2001;28:683–693. doi: 10.1016/s0969-8051(01)00233-5. [DOI] [PubMed] [Google Scholar]
- Vora MM, Dhalla M. In vivo studies of unlabeled and radioiodinated rhodamine-123. Nuc. Med. Biol. 1992;19:405–410. doi: 10.1016/0883-2897(92)90126-j. [DOI] [PubMed] [Google Scholar]
- Wallhaus TR, Lacy J, Stewart R, Bianco J, Green MA, Nayak N, Stone CK. Copper-62-pyruvaldehyde bis(N-methyl-thiosemicarbazone) PET imaging in the detection of coronary artery disease in humans. J. Nucl. Cardiol. 2001;8:67–74. doi: 10.1067/mnc.2001.109929. [DOI] [PubMed] [Google Scholar]
- Yoon YH, Jeong JM, Kim HW, Hong SH, Lee Y-S, Kil HS, Chi DY, Lee DS, Chung J-K, Lee MC. Novel one-pot one-step synthesis of 2′-[18F]fluoroflumazenil (FFMZ) for benzodiazepine receptor imaging. Nuc. Med. Biol. 2003;30:521–527. doi: 10.1016/s0969-8051(03)00030-1. [DOI] [PubMed] [Google Scholar]