Abstract
Stromal-epithelial signaling is a critical regulator of normal prostate development and has been speculated to play an equally important role in the development and progression of prostate cancer. Sonic hedgehog (Shh) and bone morphogenetic proteins (BMP-4, BMP-7), expressed by the urogenital sinus epithelium and mesenchyme exert reciprocal and coordinate effects on outgrowth of nascent prostate ducts. Over-expression of Shh in the LNCaP xenograft was shown previously to accelerate tumor growth by a paracrine mechanism. A survey of BMP regulators expressed in the developing prostate revealed increased Noggin and BMP-7 mRNA in the stromal component of Shh over-expressing xenografts. In vitro studies demonstrated that treatment of LNCaP cells with BMP-4 and BMP-7 cells induced Id-1 expression and inhibited tumor cell proliferation. The activity of BMP-4 was abrogated by co-addition of Noggin; the activity of BMP-7 was not. Quantitative analysis of BMP signaling revealed ambivalent results: decreased tumor cell expression of the BMP response gene Id-1 but increased staining for phospho-SMAD 1,5, 8. To directly test whether increased xenograft tumor growth could be explained by Noggin-mediated blockade of BMP-2/4 effects on tumor cell proliferation, we generated LNCaP xenografts containing stromal cells over-expressing Noggin. Tumor cells in these xenografts exhibited decreased Id-1 and reduced SMAD phosphorylation, but tumor growth was not altered. We conclude that tumor cell Shh expression can induce significant changes in expression of BMP ligands and inhibitors in the stromal microenvironment but that acceleration of LNCaP xenograft tumor growth by Shh over-expression cannot be attributed solely to increased Noggin expression in the tumor stroma.
Keywords: Hedgehog, Bone Morphogenetic Protein, Microenvironment, Prostate Cancer, Paracrine Signaling
INTRODUCTION
The Hedgehog (Hh) and bone morphogenetic protein (BMP) pathways regulate growth, morphogenesis and cell differentiation in a variety of tissues in the mouse embryo. Secreted Hh ligand activates a signaling cascade in responsive cells by binding to the transmembrane receptor Patched (Ptc), relieving repression of another transmembrane protein Smoothened (Smo) and activating transcription of Hh target genes by the three members of the Gli transcription factor family: Gli1, Gli2 and Gli3 (Wang et al., 2007). BMP ligands bind a type II receptor which recruits and phosphorylates a type I receptor. The type I receptor phosphorylates intracellular Smad (R-Smad) which binds a co-Smad. The Smad complex enters the nucleus where it regulates transcription of specific target genes (Ye et al., 2007a).
Shh and BMP ligands are expressed in reciprocal domains in a variety of developing structures including the gut, hair follicles, teeth, bladder, lung and prostate (Bitgood and McMahon, 1995). They sometimes exhibit inductive regulatory relationships and often exert complementary influences on morphogenesis and differentiation. In the fetal prostate, Shh acts primarily in a paracrine fashion, with ligands being expressed in the epithelium and activating Hh target gene expression in adjacent mesenchyme (Lamm et al., 2002). BMP-4 is expressed in mesenchyme and likely acts directly on both the epithelium and mesenchyme (Lamm et al., 2001). BMP-7 is expressed in both the mesenchyme and epithelium of the developing prostate (Grishina et al., 2005). Shh and BMP-4/BMP-7 exert positive and negative effects, respectively, on epithelial proliferation in the pre-natal prostate and outgrowth of epithelial buds that form the nascent prostate ducts. Increased mesenchymal expression of Noggin, a BMP antagonist, occurs at sites of ductal budding (Cook et al., 2007) and it appears that Noggin favors bud formation by altering the balance of Shh and BMP-4 activity (Vezina et al., 2008).
Recent studies suggest that Shh and BMP may exert similar, reciprocal activities in prostate cancer. BMP ligands have been shown to inhibit prostate cancer cell line proliferation and tumor growth (Brubaker et al., 2004; Tomari et al., 2005). It has been shown that expression of BMP-2, 4, and 7 ligands, BMP receptors and intracellular signaling components is diminished in prostate cancer as compared to normal tissue (Horvath et al., 2004; Kim et al., 2000; Masuda et al., 2003) and that loss of BMP-7 expression in a human prostate cancer cell line increases invasion (Ye et al., 2007b). On the other hand, increased Hh ligand expression and Hh signaling have been linked to accelerated tumor growth, progression and metastasis (Fan et al., 2004; Karhadkar et al., 2004; Sanchez et al., 2004; Sheng et al., 2004). Our laboratory has shown previously that Shh over-expression by tumor cells in the LNCaP xenograft significantly accelerates tumor growth (Fan et al., 2004). LNCaP cells are not Hh-responsive and there is no evidence of autocrine pathway activation (Zhang et al., 2007). Instead, tumor cell-derived Shh activates Hh target genes in mouse cells that comprise the stromal microenvironment of the xenograft tumor (Fan et al., 2004) and drives tumor growth by a paracrine mechanism. Here we examine the effect of tumor cell Shh over-expression on BMP signaling in the LNCaP xenograft tumor and the possible effects of altered BMP signaling on tumor growth.
RESULTS
Shh signaling alters BMP ligand and inhibitor expression in xenograft tumors
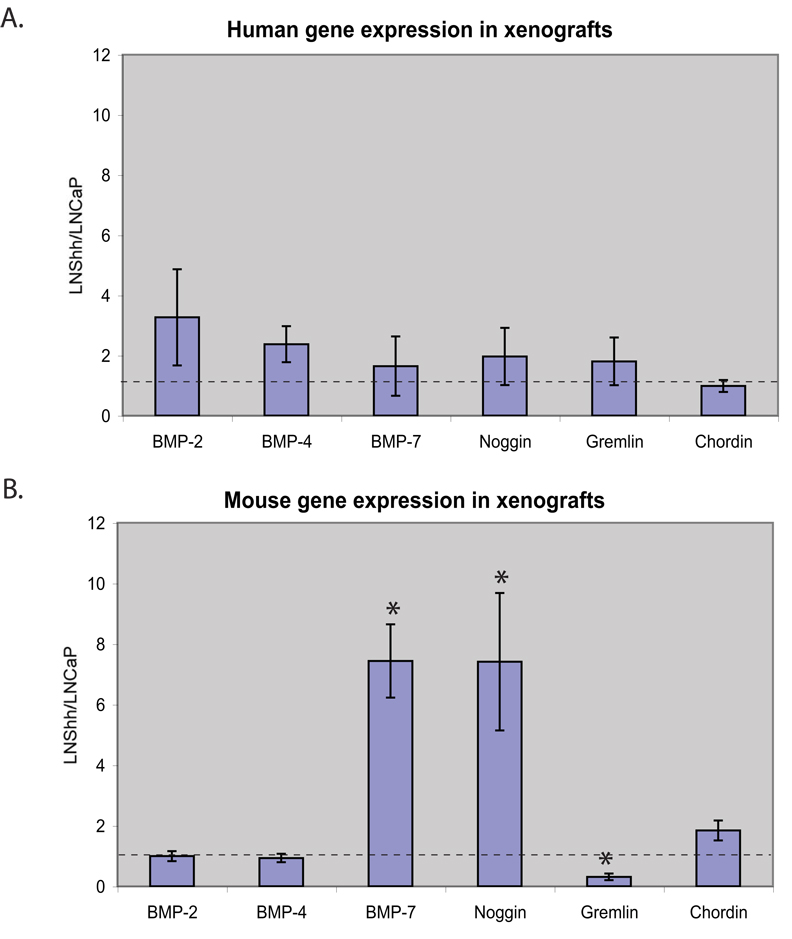
We generated prostate cancer xenografts by injection of LNCaP or LNCaP over-expressing Shh (LNShh) subcutaneously in nude mice. We then used species-specific real-time RT-PCR to compare mRNA expression in the human tumor and mouse stromal cells comprising the established xenografts at 8–10 weeks post-injection. We examined expression of those BMP ligands and inhibitors that are expressed in the developing prostate (Cook et al., 2007; Grishina et al., 2005; Lamm et al., 2001). Analysis of gene expression in the human tumor cells revealed no significant differences in the expression of the BMP ligands BMP-2, BMP-4 or BMP-7 or of the BMP antagonists Noggin, Gremlin-1 and Chordin (Figure 1A). On the other hand, analysis of BMP ligand and antagonist expression in the mouse stroma revealed significantly increased expression of BMP-7 and Noggin, and decreased expression of Gremlin (Figure 1B).
Figure 1.
BMP ligands and antagonists were measured in LNCaP and LNShh xenograft tumors by real-time RT-PCR. The charts show the relative mean expression of BMP ligands and antagonists in LNShh (n=14) as compared to LNCaP (n=9) xenografts (dashed line) using human (A) and mouse (B) species specific primers. * (p<0.05)
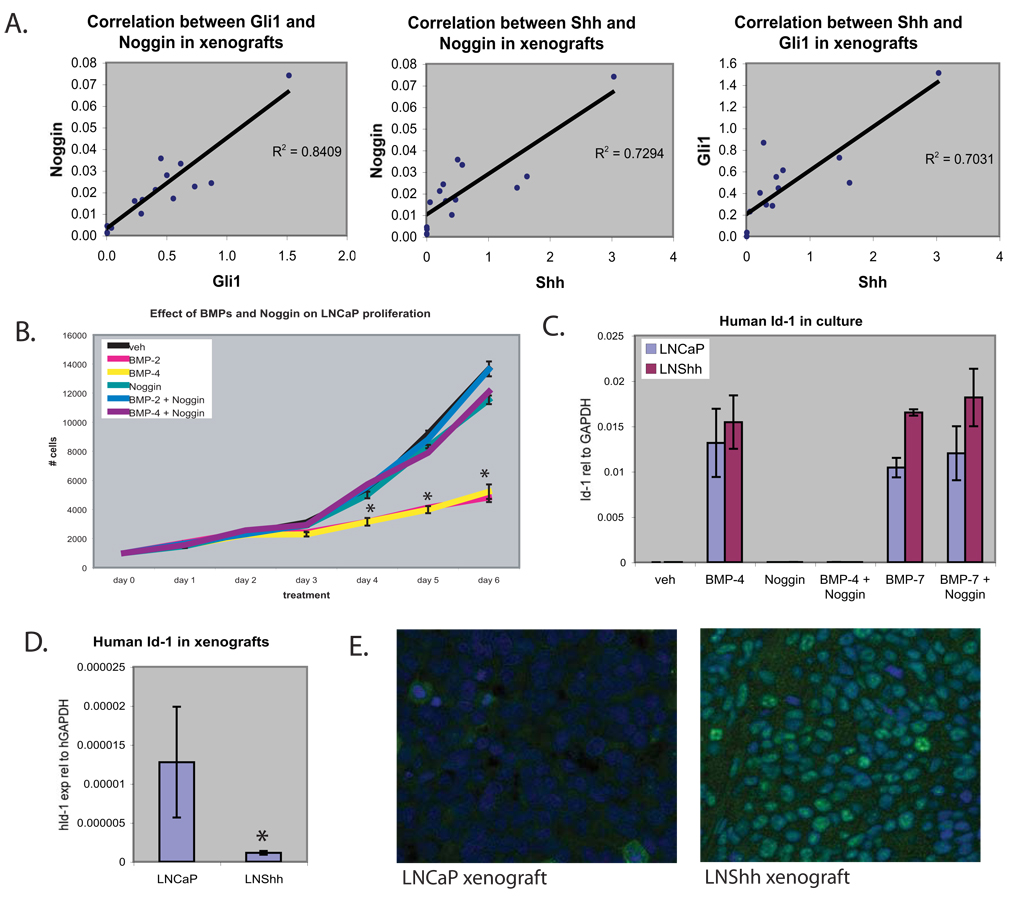
Expression of human Shh (hShh) by the LNCaP tumor cells in the Shh over-expressing xenograft tumors is mirrored by mouse Gli1 (mGli1) expression in the xenograft stroma; the significant correlation between hShh and mGli1 expression is consistent with an inductive signaling from the tumor cells to the xenograft stroma. Increased Noggin expression in the LNShh xenografts is significantly correlated with both Shh expression by the tumor cells and Gli1 expression by the tumor stroma (Figure 2A). These data indicate a specific induction of stromal Noggin expression by Hh signaling. In contrast, the increased BMP7 expression in the LNShh xenografts is not correlated with either Shh or Gli1 expression (data not shown) – arguing for an indirect mechanism of increased expression.
Figure 2.
(A) Human Shh, mouse Gli1 and mouse Noggin were measured by real-time RT-PCR (see Figure 1). The charts show correlation of human Shh, mouse Gli1 and mouse Noggin mRNA in individual LNShh xenograft tumors by linear regression. (B) LNCaP were treated with recombinant BMP and/or Noggin. Cells were treated with 0.2 ug/ml BMP-2, 20 ng/ml BMP-4 and/or 0.5 ug/ml Noggin and live cells were counted daily for 6 days (n=3). (C) Real-time RT-PCR analysis of Id-1 mRNA expression in cultured LNCaP or LNShh cells (n=4) treated with vehicle, 20 ng/ml BMP-4, 0.5 ug/ml BMP-7 and/or 0.5 ug/ml Noggin for 48 hours. * (p<0.05) different from vehicle. (D) Human Id-1 expression in LNCaP (n=9) and LNShh (n=14) xenograft tumors. Values are means +/− standard error of the mean. * (p<0.05) (E) Tumor sections were stained with phospho-Smad1, 5, 8 antibody (green) and DAPI counterstain (blue).
Noggin reverses BMP-mediated inhibition of tumor cell proliferation
Treatment with recombinant BMP-4 or the highly homologous BMP-2 each significantly inhibited LNCaP proliferation in culture (Figure 2B). A similar inhibition was observed with BMP-7 (not shown). Noggin is a BMP antagonist with relative selectivity for BMP-4 and BMP-2. Noggin alone had no effect on LNCaP proliferation, but was able to completely block the inhibitory effects of BMP-4 and BMP-2 on LNCaP proliferation (Figure 2B).
Inhibitor of differentiation (Id-1) is a helix-loop-helix protein containing a BMP-responsive element and is an early response gene to BMP in a variety of cell types (Hollnagel et al., 1999; Katagiri et al., 2002). To determine whether Id-1 expression serves as a marker of BMP activity in LNCaP, we examined Id-1 expression in cultured LNCaP cells that were treated with BMP-4 and BMP-7. Both produced a marked increase in Id-1 expression in LNCaP; the effect of BMP-4 but not BMP-7 was blocked by co-addition of Noggin (Figure 2C). To determine the net effect of Shh over-expression on BMP signaling in the xenograft tumors, we compared human Id-1 expression and SMAD phosphorylation in LNCaP and LNShh tumors. RT-PCR using human-specific primers showed Id-1 mRNA expression is reduced in LNShh xenografts (Figure 2D). Attempts to confirm this by western blot analysis of Id-1 expression were unsuccessful – possibly because of low level protein expression. The diminished expression of Id-1 mRNA suggested that increased tumor cell proliferation in the Shh over-expressing xenografts might result from increased stromal Noggin expression and blockade of growth inhibiting BMP ligands. However, immunostaining for phospho-SMAD1,5,8 surprisingly showed increased staining in the Shh over-expressing xenografts (Figure 2E).
Noggin over-expression does not increase tumor growth rate
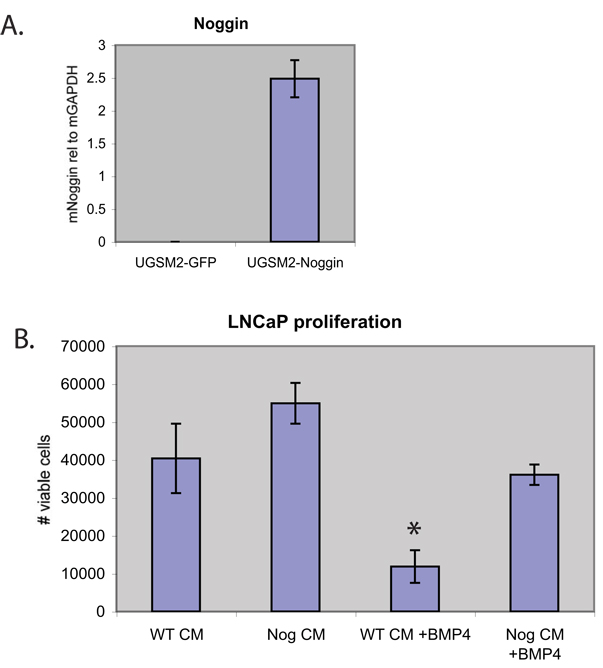
The discrepant changes in Id-1 expression and SMAD phosphorylation could be explained by the combined, differential effects of changes in Noggin, Gremlin and BMP7 expression on Id-1 expression and SMAD phosphorylation. To directly test whether Noggin-mediated blockade of BMP signaling is sufficient to accelerate xenograft tumor growth, we generated bi-clonal xenograft tumors in which Noggin was over-expressed by stromal cells. UGSM-2 cells, an immortalized prostate mesenchymal cell line (Shaw et al., 2006), were infected with an IRES-GFP retrovirus expressing mouse Noggin or the empty IRES-GFP. Noggin mRNA is highly over-expressed in the UGSM2-Noggin cell line (Figure 3A). Secretion of active Noggin protein by the UGSM2-Noggin cell line was confirmed by showing that conditioned medium (CM) from cultured UGSM2-Noggin cells but not UGSM2-GFP cells could block the inhibitory effect of BMP-4 on LNCaP proliferation (Figure 3B).
Figure 3.
(A) Real-time RT-PCR of Noggin mRNA in Noggin over-expressing stromal cells (UGSM2-Noggin, n=2) or GFP transfected cells (UGSM2-GFP, n=2). (B) Conditioned media (CM) was derived from wildtype stromal cells (UGSM2-GFP, WT CM) or Noggin-overexpressing stromal cells (UGSM2-Noggin, Nog CM). LNCaP were treated with CM alone or in combination with 20 ng/ml BMP-4 (n=2 for all groups). Live cells were counted after 2 days. Values are means +/− standard error of the mean. * (p<0.05)
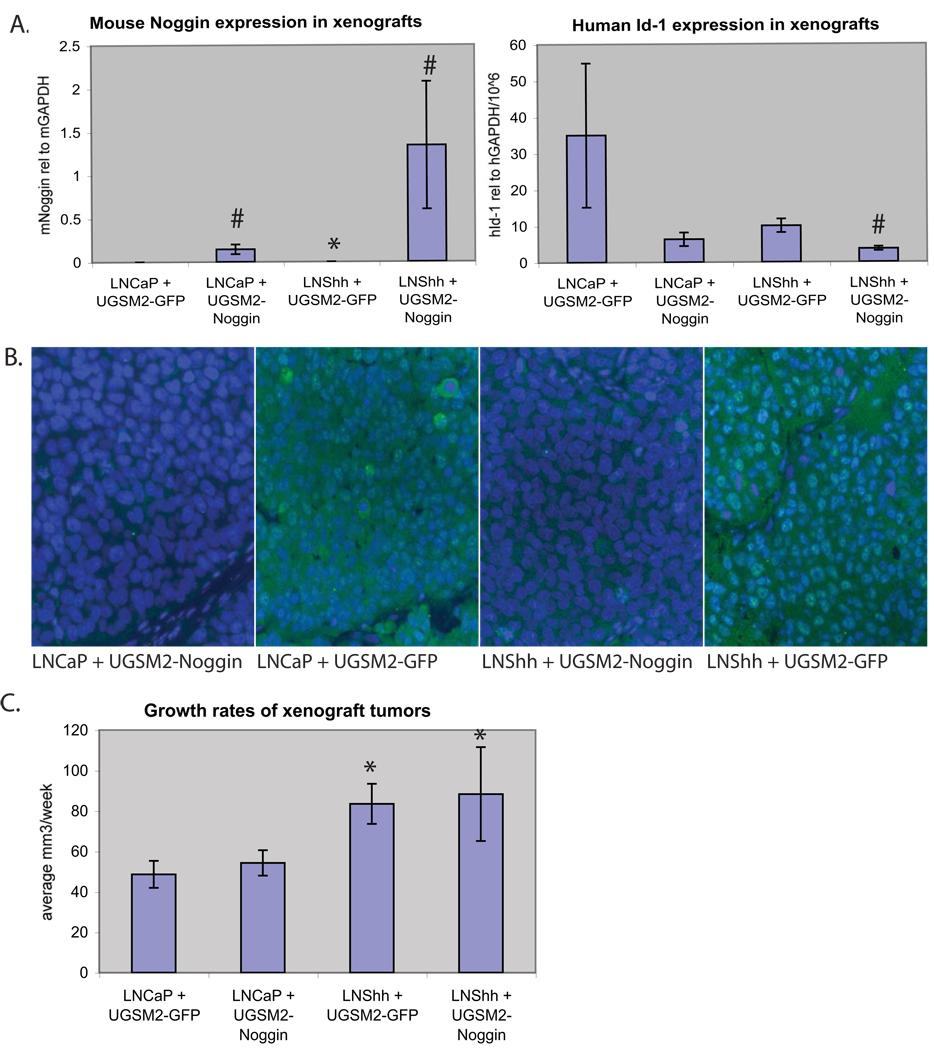
Xenografts were generated by co-injecting LNCaP cells together with UGSM2-Noggin cells or UGSM2-GFP cells. GFP expressing stromal cells could be observed in histological sections. Xenografts made with UGSM2-Noggin cells did not display any significant histologic changes in comparison to UGSM2-GFP tumors (data not shown). Over-expression of Noggin in the xenograft stroma containing UGSM2-Noggin cells decreased both Id-1 expression (Figure 4A) and SMAD phosphorylation (Figure 4B) in LNCaP. However, stromal Noggin over-expression did not accelerate growth of the LNCaP xenografts (Figure 4C). Xenograft tumors were also made by co-injecting LNShh with UGSM2-Noggin cells or control UGSM2-GFP cells. Noggin expression in the Shh over-expressing xenografts is further increased by the presence of UGSM2-Noggin cells in the xenograft stroma; this was associated with decreased Id-1 expression (Figure 4A) and SMAD phosphorylation (Figure 4B) in LNShh, but no effect on tumor growth (Figure 4C).
Figure 4.
(A) Expression of mouse Noggin (Left) or human Id-1 (Right) determined by RT-PCR using species-specific primers in LNCaP and LNShh xenograft tumors made with co-injection of either UGSM2-GFP or UGSM2-Noggin cells (n= 6–14 tumors per group). (B) Tumor sections were stained with phospho-Smad1, 5, 8 antibody (green) and DAPI counterstain (blue). (C) Growth rates shown are the averaged growth of individual tumors per week +/− standard error. * (p<0.05) compared to respective LNCaP xenografts. # (p<0.05) compared to respective UGSM2-GFP xenografts.
DISCUSSION
Hh and BMP signaling are intimately intertwined in a variety of developmental processes controlling patterning, growth and differentiation. In the developing prostate, Shh and Indian Hedgehog (Ihh) are expressed in the epithelium and activate the Hh signal transduction pathway in adjacent mesenchyme. BMP-4 and BMP-7 are both expressed primarily in the UGS mesenchyme, although BMP7 expression is also observed in the epithelium of the developing ducts (Grishina et al., 2005). Both BMP-4 and BMP-7 inhibit epithelial proliferation and reduce prostate ductal budding (Grishina et al., 2005; Lamm et al., 2001). Noggin is the primary BMP antagonist expressed in the developing prostate and can reverse the inhibitory effect of BMP-4 on both proliferation and budding (Cook et al., 2007).
The relevance of developmental signaling pathways to cancer is well established and there are many examples of errant pathway activation as a basis for tumor development or progression. The role of Hh signaling in prostate cancer, in particular, has recently become a subject of considerable interest. Studies by a number of different laboratories suggest that both autocrine and paracrine Hh signaling may contribute to tumor growth. Some studies present evidence suggesting that ligand-dependent autocrine signaling is present in prostate cancer; others support a role for ligand-independent Hh pathway activation in tumor cells. On the other hand, our studies have been consistent with a primary role for ligand-dependent paracrine signaling in prostate cancer. Stromal-epithelial interactions are known to play a critical role in tumor growth, progression and metastasis, however, the stromal-epithelial dialogue in cancer has generally not been deciphered with respect to the interplay and cross-talk of developmental signaling pathways. This is the first demonstration, to our knowledge, that Shh expression by tumor cells can up-regulate expression of a BMP antagonist in the tumor stroma. We were particularly interested to discover the increase in Noggin expression in the Shh over-expressing xenograft tumors since it hinted at a reiteration of the developmental interconnection between the Hh and BMP signaling pathways. Shh has not been shown to induce mesenchymal Noggin in the developing prostate, but precedent does exist for such an inductive relationship. Shh has been shown to induce Noggin expression in lung development (Weaver et al., 2003) and we have found the Shh can induce Noggin in freshly isolated MEFs (unpublished observations). Therefore, our observations implicate the up-regulation of Noggin expression in the tumor stroma as a paracrine response to increased tumor cell expression of Shh.
Our efforts to tease out the effect of Noggin induction on tumor growth revealed a complex interplay of nested BMP signaling effects. Whereas BMP-4 and BMP-7 could both inhibit LNCaP cell proliferation and induce an increase in Id-1 expression, only BMP-4 induced a discernable increase in SMAD phosphorylation. Noggin was able to block the effects of BMP-4 in vitro but was unable to block the effects of BMP-7. These different affects on the actions of BMP-4 and BMP-7 presumably result from differences in affinity of Noggin for the two ligands (Zimmerman et al., 1996). The Shh over-expressing tumors exhibited a disparate change in the two indicators of BMP signaling: decreased Id-1 expression and increased SMAD phosphorylation. This is best explained by the complex interplay of the changes in BMP-7, Gremlin and Noggin expression associated with Shh over-expression, but may also reflect the effect of Shh over-expression on other, unrelated signaling pathways that influence Id-1 expression and SMAD phosphorylation. Noggin over-expression in xenograft stroma did decrease Id-1 transcription and dramatically reduced SMAD phosphorylation. Even so, Noggin over-expression did not accelerate growth of either the LNCaP or the LNShh xenografts. This signifies that inhibition of BMP signaling alone is not sufficient to drive growth of the LNCaP xenograft or to further increase growth of the tumor in the context of Shh over-expression. Our studies of the transcriptional response to Shh in UGSM-2 cells has identified over 100 potential target genes; among a subgroup of 18 validated target genes are genes related to Wnt signaling, Notch signaling and angiogenesis (Yu et al., 2009). A number of these are induced in the LNShh xenograft (Shaw et al., 2009). These data suggest that the growth accelerating effect of Shh over-expression in the xenograft tumor may be multifactorial, involving several different pathways and mechanisms. Considering the multiplicity of pathways and complex interactions that regulate normal development and the likelihood that reiteration of developmental signaling in tumors involves an equivalent level of complexity, it may be naive to expect to mimic the net growth effect of a major regulator such as Shh by engineering single gene expression changes in the tumor stroma. Alternative strategies may be required that take into account the myriad of factors that influence tumor growth. One approach would be to characterize the effects of changes in expression of individual target genes and to model their composite growth effect. Another would be to determine the regulatory network defined by the stromal transcriptional response to Shh in the xenograft tumor and compare this with the mesenchymal transcriptional response in the developing prostate and to use prostate development as the growth model to decipher the mechanism for the growth promoting effect of Hh signaling in tumors.
MATERIALS AND METHODS
Xenografts
Xenograft tumors were generated in adult male CD-1 nude mice. For canonical tumors, 2×106 LNCaP or LNShh cells were mixed with an equal volume of Matrigel and injected subcutaneously on the flanks of mice. For bi-clonal tumors, 2×106 LNCaP/LNShh and 0.5×106 UGSM cells were combined with Matrigel and injected subcutaneously as described above. LNShh cells stably express full-length human Shh cDNA (Fan et al., 2004). Tumors were measured weekly with calipers and tumor volume was calculated as the volume of a spheroid using the formula: Vol = L×W×H×0.5236.
In vitro BMP, Noggin treatment of LNCaP
LNCaP cells were plated in 24-well plates at a density of 2×104 cells/well in complete media and allowed to attach for 48 hours. Then cells were treated with recombinant human BMP-2, BMP-4, BMP-7 or mouse Noggin (R&D Systems) according to the ED50 doses from the manufacturer. Cells were counted daily using a Coulter counter. There were no significant differences in cell viability with each treatment by Trypan Blue exclusion analysis. For gene expression analysis, RNA was harvested after 48 hours treatment. RNA was purified and prepared for RT-PCR as described below.
Gene expression analysis
RNA was isolated from cultured cells and tumors using RNeasy mini kit (Qiagen, Valencia, CA) with optional on-column DNase digestion to eliminate contaminating DNA. 1 ug of total RNA was reverse transcribed to generate cDNA using M-MLV reverse transcriptase (Invitrogen). Relative mRNA quantity was determined by real-time RT-PCR using iCycler instrumentation and software (BioRad, Hercules, CA).
Immunohistochemistry
Formalin-fixed paraffin embedded sections were dewaxed, rehydrated and processed for antigen retrieval 10 minutes in citrate buffer. Phospho-Smad 1, 5, 8 was stained by incubation with anti-Phospho-Smad 1, 5, 8 antibody 9511 (Cell Signaling Technology, Danvers, MA) diluted to 1 ug/ml in PBS + 10% goat serum + 1% BSA overnight at 4°C. The primary antibody was detected by incubation with anti-rabbit conjugated with Alexa 488 (Molecular Probes, Eugene, OR) at 5 ug/ml for 45 minutes at room temperature. Slides were mounted with Vectashield Hardset + DAPI mounting media (Vector, Burlingame, CA) and imaged using an Olympus model BX51 fluorescent microscope and Spot Advanced software v. 3.5.2.
Generation of Noggin over-expressing cell line
Mouse Noggin cDNA was obtained from Richard M. Harland at the University of California at Berkeley. A 950bp fragment of Noggin cDNA was directionally cloned into pRV-IRES-EGFP retroviral vector using NotI and BamHI sites. pRV-IRES-EGFP is a VSV-G pseudotyped murine leukemia viral vector that was generously provided by F. Michael Hoffmann (University of Wisconsin-Madison). UGSM-2 cells were infected with pRV-mNoggin-IRES-EGFP retrovirus and GFP expressing cells were collected by fluorescence-activated cell sorting 1 week later. Similarly, control UGSM2-GFP cells were collected following infection with empty vector pRV-IRES-EGFP. Expression of Noggin mRNA was quantitated by real-time RT-PCR. Secretion of biologically active Noggin by UGSM2-Noggin cells was verified by the ability of conditioned media from UGSM2-Noggin cells to prevent BMP-induced growth arrest of LNCaP cells in vitro.
Statistics
We analyzed tumor growth rate by obtaining slopes. These were obtained by calculating the difference between final and initial tumor volumes, and then dividing by the intervening number of weeks: (Vn−V0)/n, where V0 denotes the tumor volume (in mm3) when it first becomes apparent, Vn denotes the tumor volume n weeks later, and n is the number of weeks between tumor appearance and the end of the experiment or the week in which the animal needed to be euthanized, whichever occurred earlier. Tumors with one or more of the following conditions were excluded from the analysis: those with a final volume of less than 100mm3, those that contracted (i.e. final volume < initial volume), those that were undetectable in any given week post initial establishment, and those with a slope less than 10mm3/week. One-Way Analysis of Variance (ANOVA) was used to test for differences in growth rate (slope) due to treatment; slopes within a given animal were considered independent. If the overall F-test was significant (P < 0.05) pair-wise comparisons between treatments were examined. This procedure is equivalent to Fisher's protected least-squares differences (LSD). In order to better meet the assumptions of ANOVA, rank and logarithmic transformations of the original data were considered. Slopes for tumor growth seemed to have a slightly positive skew distribution. A logarithmic transformation did not improve matters, so ANOVA was performed on the raw slopes. We analyzed differences in gene expression by comparing the average GAPDH normalized value for each gene using a t-test assuming equal variances.
Acknowledgments
Grant sponsor: Department of Defense; grant number W81XWH-04-1-0263
AS was supported by Department of Defense predoctoral grant W81XWH-06-1-0060
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Aubie Shaw: no disclosures
Jerry Gipp: no disclosures
Wade Bushman: no disclosures
REFERENCES
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Brubaker KD, Corey E, Brown LG, Vessella RL. Bone morphogenetic protein signaling in prostate cancer cell lines. J Cell Biochem. 2004;91:151–160. doi: 10.1002/jcb.10679. [DOI] [PubMed] [Google Scholar]
- Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson RE, Bushman W. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol. 2007;312:217–230. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145:3961–3970. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Dev Biol. 2005;288:334–347. doi: 10.1016/j.ydbio.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- Horvath LG, Henshall SM, Kench JG, Turner JJ, Golovsky D, Brenner PC, O'Neill GF, Kooner R, Stricker PD, Grygiel JJ, Sutherland RL. Loss of BMP2, Smad8, and Smad4 expression in prostate cancer progression. Prostate. 2004;59:234–242. doi: 10.1002/pros.10361. [DOI] [PubMed] [Google Scholar]
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells. 2002;7:949–960. doi: 10.1046/j.1365-2443.2002.00573.x. [DOI] [PubMed] [Google Scholar]
- Kim IY, Lee DH, Ahn HJ, Tokunaga H, Song W, Devereaux LM, Jin D, Sampath TK, Morton RA. Expression of bone morphogenetic protein receptors type-IA, -IB and -II correlates with tumor grade in human prostate cancer tissues. Cancer research. 2000;60:2840–2844. [PubMed] [Google Scholar]
- Lamm ML, Catbagan WS, Laciak RJ, Barnett DH, Hebner CM, Gaffield W, Walterhouse D, Iannaccone P, Bushman W. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol. 2002;249:349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol. 2001;232:301–314. doi: 10.1006/dbio.2001.0187. [DOI] [PubMed] [Google Scholar]
- Masuda H, Fukabori Y, Nakano K, Takezawa Y, T CS, Yamanaka H. Increased expression of bone morphogenetic protein-7 in bone metastatic prostate cancer. Prostate. 2003;54:268–274. doi: 10.1002/pros.10193. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A, Gipp J, Bushman W. The Sonic Hedgehog Pathway Stimulates Prostate Tumor Growth by Paracrine Signaling and Recapture of Embryonic Gene Expression in Tumor Myofibroblasts. Cancer Research submitted. 2009 doi: 10.1038/onc.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A, Papadopoulos J, Johnson C, Bushman W. Isolation and characterization of an immortalized mouse urogenital sinus mesenchyme cell line. Prostate. 2006;66:1347–1358. doi: 10.1002/pros.20357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomari K, Kumagai T, Shimizu T, Takeda K. Bone morphogenetic protein-2 induces hypophosphorylation of Rb protein and repression of E2F in androgen-treated LNCaP human prostate cancer cells. Int J Mol Med. 2005;15:253–258. [PubMed] [Google Scholar]
- Vezina CM, Allgeier SH, Fritz WA, Moore RW, Strerath M, Bushman W, Peterson RE. Retinoic acid induces prostatic bud formation. Dev Dyn. 2008 doi: 10.1002/dvdy.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, McMahon AP, Allen BL. Shifting paradigms in Hedgehog signaling. Curr Opin Cell Biol. 2007;19:159–165. doi: 10.1016/j.ceb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol. 2003;258:169–184. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Ye L, Lewis-Russell JM, Kyanaston HG, Jiang WG. Bone morphogenetic proteins and their receptor signaling in prostate cancer. Histol Histopathol. 2007a;22:1129–1147. doi: 10.14670/HH-22.1129. [DOI] [PubMed] [Google Scholar]
- Ye L, Lewis-Russell JM, Kynaston H, Jiang WG. Endogenous bone morphogenetic protein-7 controls the motility of prostate cancer cells through regulation of bone morphogenetic protein antagonists. J Urol. 2007b;178:1086–1091. doi: 10.1016/j.juro.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Yu M, Gipp J, Yoon JW, Iannaccone P, Walterhouse D, Bushman W. Sonic hedgehog-responsive genes in the fetal prostate. J Biol Chem. 2009;284:5620–5629. doi: 10.1074/jbc.M809172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lipinski R, Shaw A, Gipp J, Bushman W. Lack of demonstrable autocrine hedgehog signaling in human prostate cancer cell lines. J Urol. 2007;177:1179–1185. doi: 10.1016/j.juro.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]