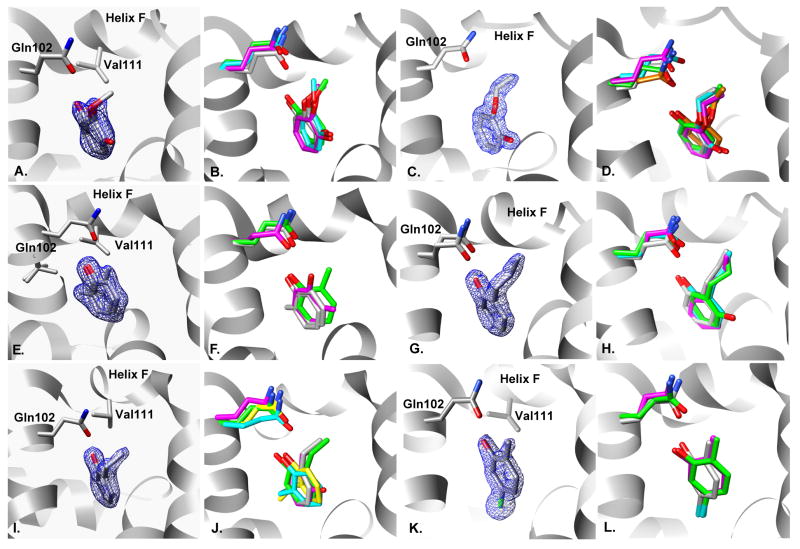
Fig 4. Comparison of predicted to experimental binding modes for the relative binding free energy predictions starting from reference compounds catechol & phenol.
A. & B. Two conformations of 2-methoxyphenol: A. X-ray result, 50:50 occupancy. 2Fo−Fc electron density map displayed at 1.0σ. B. Overlay of x-ray result (gray), prediction from catechol (green & cyan), RMSD 0.52 and 0.85Å, and prediction from phenol (magenta), RMSD 0.65 Å. C. & D. 2-ethoxyphenol: C. X-ray result,100% occupancy. 2Fo−Fc electron density map displayed at 1.5σ. D. Overlay of x-ray result (gray) and prediction from catechol (green & cyan), RMSD 0.58 and 0.76Å, and prediction from phenol (magenta), RMSD 0.86Å. E. & F. Two conformations of 2-methylphenol: E. X-ray result, 50:50 occupancy. 2Fo−Fc electron density map displayed at 1.0σ. F. Overlay of x-ray result (gray) and prediction from catechol (green), RMSD 1.02Å, and prediction from phenol (magenta), RMSD 2.03Å. G. & H. 2-propylphenol: G. X-ray result,100% occupancy. 2Fo−Fc electron density map displayed at 1.5σ. H. Overlay of x-ray result (gray) and prediction from catechol (green & cyan), RMSD 0.40 and 1.08Å, and prediction from phenol (magenta), RMSD 2.23Å. I. & J. 2-ethylphenol: I. X-ray result, 100% occupancy. 2Fo−Fc electron density map displayed at 1.5σ. J. Overlay of x-ray result (gray) and prediction from catechol (green, cyan, yellow), RMSD 0.64Å, 3.10Å, 3.08Å and prediction from phenol (magenta), RMSD 2.25Å. K. & L. 5-chloro-2-methylphenol: K. X-ray result, 100% occupancy. 2Fo−Fc electron density map displayed at 1.5σ. L. Overlay of x-ray result (gray) and prediction from catechol (green), RMSD 0.87Å, and prediction from phenol (magenta), RMSD 0.66Å.