Abstract
OBJECTIVES
To identify predictors of 30-day serious events after syncope in older adults.
METHODS
We reviewed the medical records of older adults (age≥60) who presented with syncope or near-syncope to one of three emergency departments (ED) between 2002 and 2005. Our primary outcome was occurrence of a pre-defined serious event within 30 days after ED evaluation. We employed multivariable logistic regression to identify predictors of 30-day serious events.
RESULTS
Of 3,727 potentially eligible patients, 2,871 (77%) met all eligibility criteria. We excluded an additional 287 patients who were diagnosed with a serious clinical condition in the ED. In the final study cohort (n=2,584), we identified 173 (7%) patients who experienced a 30-day serious event. High-risk predictors included age greater than 90 years, male gender, history of an arrhythmia, triage systolic blood pressure greater than 160mm HG, abnormal electrocardiogram, and abnormal troponin I level. A low risk predictor was a complaint of near-syncope rather than syncope. A risk score, generated by summing high risk predictors and subtracting the low risk predictor, can stratify patients into low (Event rate: 2.5%, 95% CI:1.4, 3.6), intermediate (Event rate: 6.3%, 95% CI:5.1, 7.5), and high (Event rate: 20%, 95% CI:15, 25) risk groups.
CONCLUSION
We identified predictors of 30-day serious events after syncope in adults aged 60 years and greater. A simple score was able to stratify these patients into distinct risk groups, and if externally validated, might have the potential to aid ED decision making.
INTRODUCTION
Background
Syncope is a common complaint among those seen in the emergency department (ED) which is responsible for 1–3% of all ED visits and hospital admissions.1–3 Because of co-morbid illnesses, concurrent medications, cognitive impairment, and age-related physiologic changes4, 5, older adults experience a higher incidence of syncope, related health-services use, and associated serious events compared to younger adults.6–10 As a result, patients aged 60 and older are often hospitalized after syncope1, and consensus guidelines suggest a lowered threshold for admission in patients of advanced age.11, 12
Existing patterns of care are nevertheless characterized by high variance13, 14 and low diagnostic and therapeutic yield. Between 39% and 50% of admitted patients are discharged without an explanation for syncope15, and in one study 60% of older patients received no specific therapies during their inpatient stay.16 Existing admission practices consume significant health resources, and the total annual costs of syncope-related admissions exceeds $2 billion.2
Importance
Improved risk prediction has the potential to safely reduce practice variation and hospitalizations. Several investigators have described predictors of serious clinical events after syncope7–9, 17–20, but these studies enrolled relatively small numbers of patients (n<800), and the clinical utility of prediction instruments has been limited by wide confidence intervals for false negative classifications (95%CI: 0–14%). Some prediction instruments focus on one year outcomes, which may not be relevant to emergency department decision making.7–9 Attempts to externally validate existing syncope instruments have yielded mixed results.21–23 Finally, several reports assess age as a dichotomous risk factor (e.g. age≥60 years)8, 9, 17, but there have been no published attempts to further risk stratify older adults who present with syncope. The lack of age-specific risk stratification represents an important gap in the literature, since syncope-related health resource use is concentrated in older adults.2
This Investigation
To address this knowledge gap, we reviewed the medical records of older adults (age≥60) who presented with syncope or near-syncope to one of three emergency departments (ED). The goal of this report is to identify predictors of 30-day serious events after syncope in such patients. To maximize sample size, data quality, and outcomes detection, all data were collected from a regional, integrated managed care system.
METHODS
Study Design and Setting
We performed a structured medical record review of patients who presented to one of three emergency departments (ED) within a regional managed care system (Kaiser Permanente Southern California-KPSC), from January 2002 to December 2005, with a complaint of syncope or near-syncope, and for whom a serious underlying cause was not apparent during the time of their ED stay. Annual ED visit volumes ranged from 39,000 to 153,000 at the three sites. All care was provided by an attending emergency physician, and none of the sites was a trauma or emergency medicine residency training center. The three sites were selected because data on ED visits, hospitalizations, and laboratory and ECG testing during the study period were routinely available through an electronic medical records system. The KPSC and UCLA IRBs approved this study.
Overview of Screening and Chart Review Methodology
Administrative discharge codes for ED visits were used to identify potentially eligible patients. Three research associates performed all chart reviews for eligibility screening, outcomes identification, and covariate abstraction. Two of the abstractors were non-physician research project managers with at least two years of experience. One of the abstractors was a foreign medical graduate. All research associates were trained on a practice set of 10 charts. Standardized abstraction forms were used for cohort screening, chart abstraction, and identification of serious outcomes (See Appendices). Variables in the abstraction forms were explicitly defined in a study code-book.
To minimize missing data, research associates reviewed available ED, admitting, and consulting notes in a hierarchical fashion, with only the ED chart being used if it provided the information being sought; if such information was missing from the ED record, the research associate would attempt to find the information in admitting or consulting notes. Any ambiguous chart data was referred to the principal investigator, who made the final coding decision in such cases. The research associates were blinded to study objectives. Research associate performance was monitored on a monthly basis by the principal investigator.
In pilot work, we assessed the validity of screening and chart abstraction methods. To assess positive and negative predictive value of discharge code screening, a physician-investigator (BCS) blinded to the ED discharge code reviewed 200 charts (100 consecutive charts with an ED discharge code consistent with syncope; and 100 consecutive charts without an ED discharge code for syncope) to assess the presence or absence of syncope.
To assess inter-rater reliability of chart review for study eligibility, outcomes identification, and covariate abstraction, all research associates independently reviewed a validation set of 100 charts. Research associate reviews were compared to reviews performed by a physician-investigator (BCS), and the physician chart review was considered the gold standard. The inter-rater reliability between research associate and physician reviews for study eligibility and covariate abstraction were estimated using the kappa statistic. We also estimated the sensitivity of research associates to identify serious outcomes.
Finally, we assessed inter-physician reliability in identifying and classifying serious events. We identified a subset of 60 charts that were flagged as potentially documenting a serious event after research associate review. Two physicians (BCS, GG) independently reviewed these charts to identify the occurrence and type of serious event.
Selection of Participants
Adult patients aged 60 or older with an ED complaint of syncope or near-syncope were eligible for enrollment. We defined syncope as a sudden, transient loss of consciousness, and near-syncope as a sensation of imminent loss of consciousness, without actual syncope.
We excluded patients who did not clearly present with syncope or near-syncope3, 8, 24, including those who presented with a generalized seizure, intoxication, no spontaneous return to baseline mental status, and patients who experienced loss of consciousness as a result of head trauma. We required patients to have regained consciousness spontaneously and excluded those who required electrical or pharmacologic treatment at initial presentation. If a patient had more than one visit for syncope during the study period, then we considered only the first visit as eligible for study inclusion. We excluded all non-members of the health plan, as we did not have post-discharge outcome information on these patients. Finally, we excluded patients in whom a serious underlying cause of the syncope was evident during the time of the index ED visit.
We identified the study cohort using a three-stage screening process. First, we identified all KPSC patients aged 60 or over with an ED visit from January 1, 2002 to December 31, 2005 with an ED discharge diagnosis of ICD-9 code 780.2 (Syncope And Collapse: Blackout; Fainting; (Near) (Pre) syncope; Vasovagal attack) using administrative data.
Second, three trained KPSC research associates reviewed ICD-9 flagged ED charts for inclusion and exclusion criteria (Appendix 1). ED visits were eligible only if there was explicit documentation of syncope or near-syncope. Other conditions, including weakness, dizziness, seizures, vertigo, and confusion, were ineligible for inclusion. All indeterminate cases of study eligibility were reviewed by a physician-investigator (BCS).
Finally, research associates identified all visits where a serious condition was diagnosed in the ED. These charts were overread by an emergency physician (see Outcomes Identification below) and excluded from further analysis.
Outcomes Definition (Appendix 2)
Our primary outcome was any predefined serious clinical event that occurred during the 30-day period after the initial ED evaluation. We classified outcomes based on the recommendations of a working group of emergency physicians, internists, geriatricians, and cardiologists who identified syncope-related conditions for which hospital admission may be beneficial.25 Serious events included death, arrhythmias, myocardial infarction, a new diagnosis of structural heart disease thought to be related to syncope, pulmonary embolism, aortic dissection, stroke/TIA, subarachnoid or non-traumatic cerebral hemorrhage, and significant hemorrhage or anemia requiring blood transfusion. Admitted patients who required any of several pre-defined cardiac interventions during their stay were also considered to have a serious outcome. Cardiac interventions included pacemaker/defibrillator insertion, coronary angioplasty, and open heart surgery for ischemic or valvular heart disease.
We used professional society guidelines26, existing arrhythmia research3, 8, 17, and input from local electrophysiologists to define clinically significant arrhythmias. These include ventricular tachycardia (VT); sinus pause greater than 3 seconds; third-degree atrioventricular block; Mobitz II atrioventricular block; symptomatic supraventricular tachycardia (heart rate greater than 100 beats per minute); or symptomatic bradycardia (heart rate less than 60 beats per minute). We subclassified ventricular tachycardia into VT terminated by an implanted defibrillator, sustained VT (duration greater than 30 seconds), and non-sustained VT (duration greater than three beats but less than 30 seconds). “Symptomatic” refers to the simultaneous occurrence of dizziness, lightheadedness, hypotension (systolic blood pressure<90), or syncope with an arrhythmia on ECG monitoring. We also include electrophysiologic findings that represent risk factors for a dangerous arrhythmia, including inducible, sustained ventricular tachycardia; H-V intervals >100 ms; symptomatic supraventricular tachycardia (SVT); infra-Hisian block; and prolonged corrected sinus node recovery time (>550 ms).
Data Collection
Outcomes Identification (Appendices 2 and 3)
We identified all deaths by linking patient records to California vital statistics files and the Social Security Death Index.27 Research associates reviewed all available medical records of study subjects to identify non-fatal serious events. Member patients receive most of their care within the regional managed care network. Member patients who are seen in a non-network ED and require hospitalization are typically transferred to a network hospital. All health resources use within the regional managed care network is captured by the electronic medical system. If an electronic transcript within the managed care network describing a health encounter was unavailable, then research associates obtained and reviewed the paper chart. All events flagged by a research associate were reviewed by a physician-investigator, who made final determination of occurrence, timing, and classification of a serious event.
Candidate Prediction Variables (Appendices 4 and 5)
We reviewed previous studies to identify potential candidate predictors for arrhythmias, sudden cardiac death, and other serious events.8, 16, 17, 28–32 Additional variables were considered based on the input of a local panel of emergency physicians, internists, a geriatrician, and a cardiologist. In pilot work, we identified variables with either high rates of missing data (e.g. documented orthostatic vital signs) or low inter-rater reliability (e.g. complaint of new neurologic symptoms) and excluded them from our final abstraction form.
Research associates used structured data forms to collect demographic, co-morbidity, symptom, exam, and test information from ED, admitting, and consultant notes. All notes were dictated by an attending physician. For co-morbidity variables, we assumed that there was no co-morbidity in the absence of supportive documentation. For symptom, physical exam, and test variables, we recorded whether the data were explicitly present, absent, or missing. If a test was not ordered by the treating physician, then the test variable was coded as missing. Research associates noted whether there was evidence of associated traumatic injury, and presence of trauma was confirmed by physician review. We defined traumatic injury as the presence of traumatic intracranial injury, long bone fracture, or thoraco-abdominal visceral injury.
For test variables, we abstracted electrocardiogram (ECG) results from cardiologist overread and classified them as normal, non-specific changes, or abnormal. The study team did not attempt to re-interpret ECGs. We did not compare these ECGs to prior ECGs. We considered the following changes to represent abnormal ECG findings: non-sinus rhythm, sinus rhythm with HR<40, Q/ST/T changes consistent with acute or chronic ischemic heart disease, abnormal conduction intervals (QRS>0.1 ms, QTc>450 ms), left or right ventricular hypertrophy, left axis deviation, and bundle branch block. We collected hematocrit and troponin I values from laboratory data systems, and considered a hematocrit value less than 30% as abnormal. We classified troponin I values greater or equal than 0.04ng/mL as abnormal. Although serum tests were performed at each study site rather than at a central facility, all EDs used similar laboratory protocols and had the same reference ranges for hematocrit and troponin I. Finally, the presence of pyuria (>5 white blood cells per high powered field) on urinalysis testing was noted by research associates and confirmed by a physician-reviewer.
Admission Rate
At the completion of all chart review, we linked the study base to administrative data to determine whether patients were admitted after their ED evaluation. Because we discovered the administrative variable for admission status was frequently inaccurate, we re-abstracted a sample of charts to estimate the overall admission rate. A study physician (BCS) reviewed charts of all patients who experienced the primary outcome and a random sample of 100 charts of patients who did not experience the primary outcome. We then used these data to calculate a weighted estimate of the overall admission rate.
Data Analysis
We generated frequencies for demographic, co-morbidity, symptom, exam, and test characteristics, overall and stratified by occurrence of a 30-day serious event. We use chi-square tests to analyze binary and categorical variables.
In exploratory bivariate analyses, we used logistic regression to assess the shape of the relationship between continuous variables and the outcome. We identified non-linear, bivariate relationships between the outcome and age, triage systolic blood pressure and triage pulse. There was a step increase in risk associated with age>90 years, and high and low extremes of blood pressure and pulse were associated with increased risk. We converted these continuous predictors to categorical variables, and we used the results of exploratory bivariate analyses to identify cutpoints for these conversions.
To identify independent predictors of the primary outcome, we performed multivariable logistic regression. We coded all missing data as representing absence of the variable. We then used variable-specific binary indicators to flag missing data. In exploratory work, we generated a “complete” model that included all predictor variables. We then created a “reduced” model using a backward selection algorithm that retained variables at a threshold of p<0.15. We found no important qualitative differences in coefficient values or p-values between the complete and reduced models, and we present the reduced model to improve interpretability.
We employed bootstrapping methods to assess the stability of the reduced model. Using random sampling from actual study patients, we generated 1000 hypothetical study populations of equivalent size to the original cohort. We estimated coefficient point estimates using the reduced model for each hypothetical population. We estimated the bootstrapped effect size and 95% confidence intervals for each coefficient. We assessed goodness-of-fit using the Hosmer-Lemeshow test.
We assessed two different weighting schemes to generate a risk score from significant variables identified by regression modeling. These included weighting by regression coefficients rounded to the nearest integer and simple summation of the presence or absence of each variable. Receiver-operating-characteristics (ROC) curve and area-under-the-ROC-curve (AUC) for each scheme was generated using bootstrap methods. The 95% confidence intervals of the AUC and differences in AUC between the weighting schemes were obtained based on 1000 bootstrap samples.
Data management and statistical analyses were conducted using SAS software, version 9.1 (SAS Institute Inc, Cary, NC) and the publicly available statistical software R (R Development Core Team, 2008).
RESULTS
Validation of Screening and Chart Review Methodology
Compared to blinded physician chart review, ICD-9 screening demonstrated a positive predictive value of 92% (n=100 consecutive ED charts with a positive ICD-9 screen) and a negative predictive value of 100% (n=100 consecutive ED charts with a negative ICD-9 screen) for identifying patients with syncope or near-syncope.
In a validation sub-sample of 100 selected charts, research associate review for study eligibility demonstrated good inter-rater reliability (K=0.64; absolute agreement 84%) compared to blinded physician chart review. Research associates demonstrated high inter-rater reliability (K= 0.5 – 0.9; absolute agreement 73%–96%) on all elements of chart abstraction when compared to blinded physician review. Research associates were 100% sensitive in identifying serious events when compared to blinded physician review.
To assess reliability of physician over-reads, two physicians independently reviewed a sub-sample of 60 consecutive charts flagged by research associates as potentially documenting a serious event. Agreement of physician reviewers was high for the occurrence of serious events (K=0.8; absolute agreement 90%), and there was complete agreement in event classification.
Cohort Characteristics
We identified 3,727 patients aged 60 or older with an ED discharge diagnosis of syncope. We excluded 321 non-member patients. Of the remaining 3,406 patients, 2972 had available medical chart information and explicit documentation of syncope or near-syncope. We excluded 101 patients who had documentation of ongoing confusion (n=34), witnessed seizure (n=22), loss-of-consciousness after head trauma (n=16), or need for electrical or pharmacologic intervention to restore consciousness (n=32); some patients met more than one exclusion criterion. There were 2871 (84%) patients without any exclusion criteria. Of these patients, we excluded an additional 287 patients who were diagnosed with a serious condition during the ED visit.
The final study cohort includes 2,584 patients, and study sample characteristics are presented in Table 1. The mean age of the cohort was 75, with a range of 60 to 102 years. The estimated overall admission rate was 43%, and 86% of patients who experienced the primary outcome were admitted after the initial ED evaluation. Of patients with an abnormal troponin level, the median troponin I value was 0.07 ng/mL with an interquartile range of 0.04–0.2 ng/mL.
Table 1.
Study Sample Characteristics
| Characteristic | Overall Cohort [n=2584] | 30-Day Serious Event [n=173] | No 30-Day Serious Event [n=2411] | Missing Data |
|---|---|---|---|---|
| Demographics | ||||
| Age* | ||||
| 60–69 | 32% | 22% | 33% | |
| 70–79 | 38% | 38% | 38% | |
| 80–89 | 26% | 32% | 26% | |
| >90 | 4% | 9% | 4% | |
| Male* | 46% | 59% | 41% | |
| Non-White* | 20% | 12% | 21% | 7% |
| Hispanic | 12% | 10% | 12% | 7% |
| Co-Morbidities | ||||
| Coronary Artery Disease | 22% | 25% | 21% | |
| Congestive Heart Failure** | 9% | 17% | 8% | |
| Ejection Fraction <40%* | 1% | 6% | 1% | |
| Aortic Stenosis | 1% | 1% | 1% | |
| Arrhythmia* | 13% | 30% | 12% | |
| Pacemaker/AICD | 5% | 8% | 5% | |
| Stroke | 15% | 20% | 15% | |
| Prior Syncope in 30 Days | 2% | 2% | 2% | |
| Diabetes | 20% | 23% | 20% | |
| Hypertension | 61% | 61% | 62% | |
| Dementia | 9% | 13% | 9% | |
| Symptoms | ||||
| Near-Syncope*† | 31% | 20% | 32% | |
| Chest Pain | 7% | 11% | 7% | 16% |
| Shortness of Breath | 8% | 13% | 7% | 32% |
| No Prodromal Symptoms** | 16% | 23% | 16% | 13% |
| Physical Exam | ||||
| Triage Vital Signs | ||||
| HR<60** | 18% | 17% | 17% | 11% |
| HR 60–100 | 73% | 70% | 76% | |
| HR>100 | 7% | 13% | 7% | |
| SBP<90 | 2% | 3% | 2% | 11% |
| SBP 90–160 | 78% | 72% | 78% | |
| SBP>160 | 20% | 26% | 19% | |
| Cardiac Murmur | 11% | 14% | 11% | 12% |
| Abnormal Neurologic Exam | 4% | 6% | 4% | 15% |
| Major Traumatic Injury* | 4% | 8% | 3% | |
| Tests | ||||
| Abnormal ECG* | 51% | 71% | 50% | 5% |
| Non-sinus Rhythm* | 11% | 10% | 26% | |
| ST/T Changes* | 23% | 23% | 32% | |
| Abnormal Intervals* | 8% | 8% | 16% | |
| Ventricular Hypertrophy | 10% | 9% | 12% | |
| Left Axis Deviation | 14% | 14% | 15% | |
| Left Bundle Branch Block | 4% | 4% | 4% | |
| Right Bundle Branch Block | 7% | 7% | 11% | |
| Hematocrit<30 | 5% | 5% | 5% | 14% |
| Pyuria | 7% | 5% | 7% | |
| Abnormal Troponin* | 11% | 25% | 10% | 30% |
| Admitted*‡ | 43% | 86% | 40% | |
Comparison between serious event and no-serious event groups: p<0.01
Comparison between serious event and no-serious event groups: p<0.05
Compared to Syncope
Weighted estimate- see methods section
Table 2 describes the categories and frequencies of 30-day serious events, both diagnosed in the ED and in the study cohort. There were 173 patients (7% of the final study cohort) who were diagnosed with a serious condition within 30 days after their initial ED evaluation. An arrhythmia was the most common cause of a serious event, as diagnosed both in the ED and after the ED evaluation. Gastrointestinal hemorrhage/anemia requiring transfusion was the second most common serious condition detected in the emergency department, but this was rarely diagnosed after the ED evaluation.
Table 2.
Distribution of Serious Events*
| Serious Event Type | Serious Event Identified DURING ED Evaluation† n (%) | 30-Day Serious Event Occurred AFTER ED Evaluation ╪ n (%) |
|---|---|---|
| Any | 287 (10%) | 173 (7%) |
| Death | 0 (0%) | 41 (1%) |
| Cardiac Event | 198 (7%) | 120 (4%) |
| Arrhythmia | 175 (6%) | 92 (3%) |
| Ventricular Tachycardia (VT) | 25 (0.9%) | 10 (0.4%) |
| VT Terminated by AICD | 10 (0.3%) | 0 (0%) |
| Sustained VT | 5 (0.2%) | 2 (0.1%) |
| Non-Sustained VT | 10 (0.3%) | 8 (0.3%) |
| Symptomatic PSVT | 9 (0.3%) | 6 (0.2%) |
| Symptomatic Atrial Fibrillation/Flutter with Rapid Ventricular Response | 41 (1.4)% | 18 (0.7%) |
| Sick Sinus Syndrome/Sinus Pause | 23 (0.8%) | 30 (1%) |
| Third or Mobitz II Heart Block | 15 (0.5%) | 9 (0.4%) |
| Symptomatic Bradycardia | 76 (2.6%) | 20 (0.8%) |
| Abnormal Electrophysiology Study | 2 (0.1%) | 5 (0.2%) |
| Myocardial Infarction | 14 (0.5%) | 6 (0.2%) |
| Cardiac Procedure | 0 (0%) | 54 (2%) |
| Pacemaker | 40 (2%) | |
| Implantable Defibrillator | 7 (4%) | |
| Coronary Angioplasty | 3 (4%) | |
| Coronary Bypass Surgery | 4 (4%) | |
| Structural Heart Disease | 5 (0.2%) | 10 (0.4%) |
| Pulmonary Embolism | 7 (0.2%) | 6 (0.3%) |
| Stroke/TIA | 9 (0.3%) | 11 (0.4%) |
| GI Bleed/Anemia | 85 (3%) | 8 (0.3%) |
| Subarachnoid Hemorrhage | 1 (0.03%) | 0 (0%) |
Some patients experienced more than one event
These patients were excluded from further analysis;
Denominator includes 2,871 patients who met initial eligibility criteria
Denominator includes 2,584 patients in the study cohort
Of patients who were admitted at the index ED evaluation, a serious condition was identified during the initial hospitalization in 124 patients. An additional 26 patients were initially hospitalized and discharged without a serious outcome, but later were re-hospitalized with a 30-day serious outcome or experienced out-of-hospital death. There were 23 patients who were discharged after the initial ED evaluation and later were re-hospitalized with a 30-day serious outcome or experienced out-of-hospital death.
Derivation of a Syncope Risk Score
Adjusted odds ratios from a reduced, multivariable logistic regression model are presented in Table 3. There were no qualitative differences when the model included indicators for missing data. There were no qualitative changes to the model with bootstrapping techniques. Using predetermined significance thresholds, we identified six variables associated with increased risk, including: age greater than 90 years, male gender, history of an arrhythmia, triage systolic blood pressure greater than 160, an abnormal electrocardiogram, and an abnormal troponin I level. A complaint of near-syncope, compared to syncope, was associated with decreased risk.
Table 3.
Multivariate Regression Model for 30-Day Serious Events After ED Evaluation
| Variable | β Coefficient | Bootstrapped OR | Bootstrapped 95% CI |
|---|---|---|---|
| Age Greater than 90* | 0.85 | 2.3 | 1.2, 4.4 |
| Male Gender | 0.6 | 1.8 | 1.3, 2.6 |
| Near Syncope** | −0.62 | 0.5 | 0.3, 0.8 |
| Ejection Fraction <40% | 0.72 | 2.0 | 0.8, 5.0 |
| History of Arrhythmia | 0.87 | 2.4 | 1.6, 3.6 |
| Complaint of Chest Pain | 0.47 | 1.6 | 0.9, 2.8 |
| Complaint of Shortness of Breath | 0.41 | 1.5 | 0.8, 2.6 |
| Triage Systolic Blood Pressure <90 | 0.45 | 1.6 | 0.9, 2.6 |
| Triage Systolic Blood Pressure >160 | 0.49 | 1.6 | 1.1, 2.4 |
| Triage Pulse > 100 | 0.5 | 1.6 | 0.9, 2.8 |
| Major Traumatic Injury | 0.64 | 1.8 | 0.8, 4.2 |
| Abnormal Electrocardiogram | 0.65 | 1.9 | 1.3, 2.8 |
| Abnormal Troponin I (≥0.04 ng/mL) | 0.63 | 1.9 | 1.2, 2.9 |
| Likelihood ratio test for model: Chi-square=124; p<0.0001 | |||
| c-statistic: 0.73 | |||
| Hosmer Lemeshow goodness-of-fit statistic= 5.6; p=0.7 | |||
Reference Group: Age Less than 90
Reference Group: Syncope
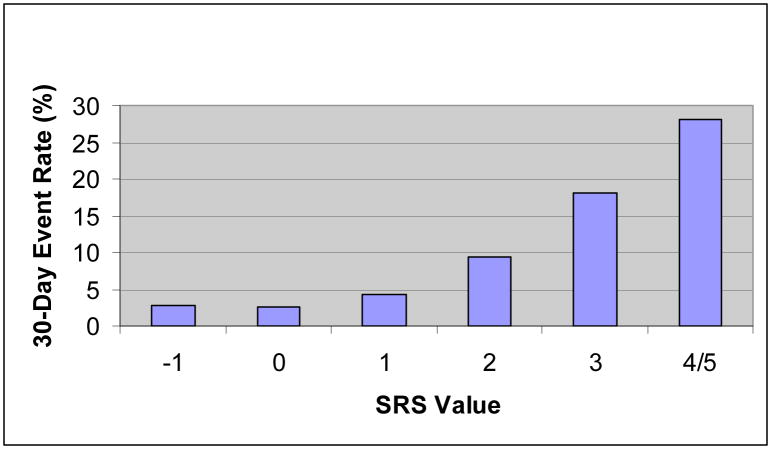
These seven variables were then used to construct a syncope risk score. Different weighting schemes to generate a risk score yielded equivalent AUCs: (weighting by rounded regression coefficients- AUC:0.61, 95%CI: 0.57,0.65; simple summation- AUC:0.61, 95%CI: 0.57,0.66). A simplified syncope risk score can be calculated by summing high risk factors and subtracting the low risk variable (Figure 1). There is a linear increase in risk with higher values of the syncope risk score (Figure 2). The syncope risk score can discriminate patients into low, medium, and high risk groups (Table 4) with a ten-fold range of risk and non-overlapping 95% confidence intervals.
Figure 1.

Calculating a Syncope Risk Score
Figure 2.
Syncope Risk Score (SRS)
Table 4.
Syncope Risk Score (SRS)
| Risk Category | SRS Value | Proportion of Patients | 30-Day Risk (95%CI) |
|---|---|---|---|
| Low | −1, 0 | 31% | 2.5% (1.4, 3.6) |
| Intermediate | 1, 2 | 58% | 6.3% (5.1, 7.5) |
| High | 3 – 6 | 11% | 20% (15, 25) |
LIMITATIONS
Strengths of this study include the relatively large cohort size, exclusion of events diagnosed during the ED evaluation, and stability of predictor-outcome associations to a number of internal validation techniques. Chart abstraction was performed by highly trained research associates, and we performed extensive assessments of inter-rater reliability; these should mitigate threats to the reliability of data derived from chart review. There are nevertheless potential limitations inherent to any retrospective study.
Data elements were not prospectively collected, and missing data may introduce bias into our results. For example, there may be selection bias by patient acuity for tests such as ECG and troponin, and this may potentially inflate the importance of such tests. We attempted to minimize missing data by using information from multiple provider notes. We found no qualitative differences when regression modeling was performed with and without controls for missing data, although future validation studies should minimize missing information through standardized testing data collection.
Patients in our study are all enrolled in a managed healthcare system, and provider practices and patient characteristics may differ from other settings. Of note, the estimated admission rate in this sample (43%) is lower than the age-matched rates reported from a national ED sample (54%)1 and a single academic center (85%).25 It is possible that discharged patients experienced undiagnosed arrhythmias which may have been identified had they been admitted. As a consequence we may be underestimating the number of patients with causes of syncope that could be potentially identified during an inpatient admission, and in turn could be misestimating the utility of individual risk criteria. Future validation studies should mitigate these limitations by standardizing duration of cardiac monitoring and include patients in non-managed care settings.
Although we defined a low risk group with a 2.5% frequency of 30-day risk for serious events, the optimum risk threshold for discharging patients is undefined. It may be possible that a ‘no-risk’ group of older patients with syncope cannot be reliably identified. Similar to our findings, other investigators have identified ‘low’ risk patients with a 2–4% frequency of syncope-related events.17, 28 One possible approach to identify an appropriate risk threshold for discharge is to determine the baseline 30-day serious event rate in an age, gender, and co-morbidity matched population of patients without syncope, although this analysis is beyond the scope of this current study.
We calculated a weighted estimate of the hospital admission rate, and we do not have complete admission data on all cohort patients because of problems with administrative data. This limits our ability to assess the hypothetical impact on admission rates if varying thresholds of the syncope risk score were used to admit or discharge patients. It is likely that the impact of the syncope risk score will vary by setting and be strongly associated with baseline clinical practices and admission rates. Future validation studies should prospectively collect disposition data.
Finally, this study is unable to assess whether patients at risk for a serious event will benefit from immediate hospitalization. For example, it is possible that some 30-day serious events were not related to the initial episode of syncope or would have occurred regardless of hospital admission (e.g. cancer related mortality). Future interventional trials of inpatient evaluation compared to a rapid, standardized ED observation protocol may clarify the benefit of admission in risk-stratified patients.
DISCUSSION
Professional society guidelines12, 15, 33 suggest that most patients who are younger than 60 years without an obvious treatable cause of syncope, or evidence of cardiac or electrocardiogram abnormality can be managed as an outpatient. There is a dearth of evidence, on the other hand, to guide the evaluation of older adults. If externally validated, our prediction instrument may supplement clinical decision-making in this group. All elements of the syncope risk score can be rapidly and reliably measured during an ED evaluation. We characterized 31% of study cohort patients as ‘low-risk’ with a 2.5% 30-day serious event rate, and these patients may be candidates for discharge or a brief observation unit evaluation. In contrast, ‘high-risk’ patients had a 20% 30-day serious event rate, and inpatient evaluation should be considered for this group. The impact of applying the syncope risk score will likely vary by practice setting and baseline admission rates, and future studies should assess this prediction instrument in different ED environments. The syncope risk score may also be used as a case-mix adjustment measure in future observational and interventional studies.
Cardiac events, and particularly arrhythmias, represented the majority of serious outcomes. Increased age8, 17, male gender19, 34, history of arrhythmia8, 17, and an abnormal ECG8, 9, 17, 19, 28 have previously been found to be associated with increased risk of a cardiac event. Although several studies have questioned the routine ordering of cardiac enzymes32, 35, we found that an abnormal troponin I level was associated with serious outcomes, even after controlling for electrocardiogram abnormalities. In our cohort, the majority of abnormal troponin I levels were in an indeterminate range between 0.04 ng/mL and 0.2 ng/mL. Elevated systolic blood pressure may either reflect poorly controlled hypertension or adrenergic surge related to an underlying serious condition. Finally, we report the novel finding that near-syncope is less frequently associated with a serious outcome as compared to syncope.
At least three other groups have derived predictors of short term events after syncope, although none have specifically studied older adults. A multi-site Italian cohort enrolled 676 ED patients with a mean age of 59 years, and 41 patients experienced 10-day mortality or required a major therapeutic procedure.19 Correlates of 10-day outcomes included an abnormal electrocardiogram, concurrent trauma, lack of prodromal symptoms, and male gender. The San Francisco Syncope Rule investigators enrolled 684 ED patients with a mean age of 62, and 79 patients experienced a 7-day serious outcome including those that were diagnosed in the ED. Predictors of 7-day outcomes included an abnormal electrocardiogram, a complaint of shortness of breath, hematocrit less than 30%, systolic blood pressure less than 90 mmHg, and a history of congestive heart failure.28 Finally, a single center Swiss study enrolled 175 patients with a mean age of 66, and 30 patients were diagnosed with an arrhythmia during an inpatient evaluation.17 Predictors of arrhythmias included age greater than 60 years, an abnormal electrocardiogram, and a history of congestive heart failure.
Discrepancies between the findings of our study and prior investigations may be in part attributable to differences in cohort age and outcomes definitions. Prior studies suggest that symptoms are poorly predictive of outcomes36, particularly among the elderly.37 A history of congestive heart failure has been associated with increased mortality after syncope.8, 38 However, Kapoor and colleagues reported a negative interaction effect between increasing age and a history of congestive heart failure for predicting death.10 This finding suggests that the age-stratified mortality risk associated with congestive heart failure for older patients is smaller compared to younger patients. Finally, routine hematocrit testing may be routinely indicated to identify older patients with gastrointestinal hemorrhage or severe anemia.28 In our study cohort, however, this test appears to have limited power to identify patients who will experience a serious event that is not diagnosed in the emergency department.
In summary, we identified seven predictors of 30-day serious events after syncope in adults aged 60 and older. A simple score was able to stratify the patients we studied into distinct low, intermediate, and high risk groups with a 10-fold difference in serious event rates. If validated in an external cohort, this syncope risk score would have the potential as an aid to clinical decision-making. Low risk patients may be candidates for discharge or a brief observation unit evaluation, whereas high risk patients may benefit from evaluation in a cardiac monitored setting. The syncope risk score can also be used as a case-mix adjustment measure for future interventional studies of syncope to assess the relative benefits of inpatient evaluation compared to a rapid, standardized ED observation protocol.
Acknowledgments
Dr. Sun is supported by a UCLA National Institute of Aging K12 award (K12AG001004) and an American Geriatrics Society Dennis Jahnigen Career Development Award (20051687). Dr. Sun received support from the UCLA Older Americans Independence Center, NIH/NIA Grant P30-AG028748, and the content does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. Drs. Mangione and Moore received support from the Resource Centers for Minority Aging Research/Center for Health Improvement of Minority Elderly (RCMAR/CHIME) funded by National Institutes of Health/National Institute on Aging (P30 AG021684) and from the UCLA/Drew Project EXPORT funded by the National Institutes of Health/National Center for Minority Health and Health Disparities (P20 MD000182).”
Footnotes
Reprints not available from the authors.
Preliminary findings from this study were presented at the American Geriatric Society Annual Scientific Meeting (Chicago, IL; May 2009) and the Society for Academic Emergency Medicine Annual Meeting (New Orleans, LA; May 2009)
References
- 1.Sun BC, Emond JA, Camargo CA., Jr Characteristics and admission patterns of patients presenting with syncope to u.s. Emergency departments, 1992–2000. Acad Emerg Med. 2004 Oct;11(10):1029–1034. doi: 10.1197/j.aem.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Sun BC, Emond JA, Camargo CA., Jr Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. 2005 Mar 1;95(5):668–671. doi: 10.1016/j.amjcard.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med. 1983;309(4):197–204. doi: 10.1056/NEJM198307283090401. [DOI] [PubMed] [Google Scholar]
- 4.Lipsitz LA, Pluchino FC, Wei JY, Rowe JW. Syncope in institutionalized elderly: the impact of multiple pathological conditions and situational stress. J Chronic Dis. 1986;39(8):619–630. doi: 10.1016/0021-9681(86)90187-6. [DOI] [PubMed] [Google Scholar]
- 5.Lipsitz LA, Wei JY, Rowe JW. Syncope in an elderly, institutionalised population: prevalence, incidence, and associated risk. Q J Med. 1985 Apr;55(216):45–54. [PubMed] [Google Scholar]
- 6.Chen LY, Shen WK, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Prevalence of syncope in a population aged more than 45 years. Am J Med. 2006 Dec;119(12):1088, e1081–1087. doi: 10.1016/j.amjmed.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002 Sep 19;347(12):878–885. doi: 10.1056/NEJMoa012407. [DOI] [PubMed] [Google Scholar]
- 8.Martin TP, Hanusa BH, Kapoor WN. Risk stratification of patients with syncope. Ann Emerg Med. 1997;29(4):459–466. doi: 10.1016/s0196-0644(97)70217-8. [DOI] [PubMed] [Google Scholar]
- 9.Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J. 2003;24(9):811–819. doi: 10.1016/s0195-668x(02)00827-8. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor W, Snustad D, Peterson J, Wieand HS, Cha R, Karpf M. Syncope in the elderly. Am J Med. 1986 Mar;80(3):419–428. doi: 10.1016/0002-9343(86)90716-3. [DOI] [PubMed] [Google Scholar]
- 11.Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;127(1):76–86. doi: 10.7326/0003-4819-127-1-199707010-00014. [DOI] [PubMed] [Google Scholar]
- 12.Huff JS, Decker WW, Quinn JV, et al. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with syncope. Ann Emerg Med. 2007 Apr;49(4):431–444. doi: 10.1016/j.annemergmed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Disertori M, Brignole M, Menozzi C, et al. Management of patients with syncope referred urgently to general hospitals. Europace. 2003 Jul;5(3):283–291. doi: 10.1016/s1099-5129(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 14.Roos NP, Wennberg JE, McPherson K. Using diagnosis-related groups for studying variations in hospital admissions. Health Care Financ Rev. 1988 Summer;9(4):53–62. [PMC free article] [PubMed] [Google Scholar]
- 15.Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 1: Value of history, physical examination, and electrocardiography. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med. 1997;126(12):989–996. doi: 10.7326/0003-4819-126-12-199706150-00012. [DOI] [PubMed] [Google Scholar]
- 16.Getchell WS, Larsen GC, Morris CD, McAnulty JH. Epidemiology of syncope in hospitalized patients. J Gen Intern Med. 1999;14(11):677–687. doi: 10.1046/j.1525-1497.1999.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarasin FP, Hanusa BH, Perneger T, Louis-Simonet M, Rajeswaran A, Kapoor WN. A risk score to predict arrhythmias in patients with unexplained syncope. Acad Emerg Med. 2003 Dec;10(12):1312–1317. doi: 10.1111/j.1553-2712.2003.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 18.Quinn J, McDermott D, Stiell I, Kohn M, Wells G. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med. 2006 May;47(5):448–454. doi: 10.1016/j.annemergmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Costantino G, Perego F, Dipaola F, et al. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol. 2008 Jan 22;51(3):276–283. doi: 10.1016/j.jacc.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 20.Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to general hospital: the EGSYS score. Heart. 2008 Jun 2; doi: 10.1136/hrt.2008.143123. [DOI] [PubMed] [Google Scholar]
- 21.Reed MJ, Newby DE, Coull AJ, Jacques KG, Prescott RJ, Gray AJ. The Risk stratification Of Syncope in the Emergency department (ROSE) pilot study: a comparison of existing syncope guidelines. Emerg Med J. 2007 Apr;24(4):270–275. doi: 10.1136/emj.2006.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birnbaum A, Esses D, Bijur P, Wollowitz A, Gallagher EJ. Failure to Validate the San Francisco Syncope Rule in an Independent Emergency Department Population. Ann Emerg Med. 2008 Feb 16; doi: 10.1016/j.annemergmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Sun BC, Mangione CM, Merchant G, et al. External validation of the San Francisco Syncope Rule. Ann Emerg Med. 2007 Apr;49(4):420–427. 427, e421–424. doi: 10.1016/j.annemergmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Sarasin FP, Louis-Simonet M, Carballo D, et al. Prospective evaluation of patients with syncope: a population-based study. Am J Med. 2001 Aug 15;111(3):177–184. doi: 10.1016/s0002-9343(01)00797-5. [DOI] [PubMed] [Google Scholar]
- 25.Sun BC, Hoffman JR, Mangione CM, Mower WR. Older age predicts short-term, serious events after syncope. J Am Geriatr Soc. 2007 Jun;55(6):907–912. doi: 10.1111/j.1532-5415.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 26.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) Circulation. 2002 Oct 15;106(16):2145–2161. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 27.Quinn J, McDermott D, Kramer N, et al. Death after emergency department visits for syncope: how common and can it be predicted? Ann Emerg Med. 2008 May;51(5):585–590. doi: 10.1016/j.annemergmed.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004 Feb;43(2):224–232. doi: 10.1016/s0196-0644(03)00823-0. [DOI] [PubMed] [Google Scholar]
- 29.Krol RB, Morady F, Flaker GC, et al. Electrophysiologic testing in patients with unexplained syncope: clinical and noninvasive predictors of outcome. J Am Coll Cardiol. 1987 Aug;10(2):358–363. doi: 10.1016/s0735-1097(87)80019-0. [DOI] [PubMed] [Google Scholar]
- 30.Denes P, Uretz E, Ezri MD, Borbola J. Clinical predictors of electrophysiologic findings in patients with syncope of unknown origin. Arch Intern Med. 1988 Sep;148(9):1922–1928. [PubMed] [Google Scholar]
- 31.Georgeson S, Linzer M, Griffith JL, Weld L, Selker HP. Acute cardiac ischemia in patients with syncope: importance of the initial electrocardiogram. J Gen Intern Med. 1992 Jul-Aug;7(4):379–386. doi: 10.1007/BF02599151. [DOI] [PubMed] [Google Scholar]
- 32.Grossman SA, Van Epp S, Arnold R, et al. The value of cardiac enzymes in elderly patients presenting to the emergency department with syncope. J Gerontol A Biol Sci Med Sci. 2003 Nov;58(11):1055–1058. doi: 10.1093/gerona/58.11.m1055. [DOI] [PubMed] [Google Scholar]
- 33.Brignole M, Alboni P, Benditt D, et al. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J. 2001 Aug;22(15):1256–1306. doi: 10.1053/euhj.2001.2739. [DOI] [PubMed] [Google Scholar]
- 34.Freed LA, Eagle KA, Mahjoub ZA, et al. Gender differences in presentation, management, and cardiac event-free survival in patients with syncope. Am J Cardiol. 1997 Nov 1;80(9):1183–1187. doi: 10.1016/s0002-9149(97)00637-1. [DOI] [PubMed] [Google Scholar]
- 35.Link MS, Lauer EP, Homoud MK, Wang PJ, Estes NA., 3rd Low yield of rule-out myocardial infarction protocol in patients presenting with syncope. Am J Cardiol. 2001 Sep 15;88(6):706–707. doi: 10.1016/s0002-9149(01)01825-2. [DOI] [PubMed] [Google Scholar]
- 36.Oh JH, Hanusa BH, Kapoor WN. Do symptoms predict cardiac arrhythmias and mortality in patients with syncope? Arch Intern Med. 1999 Feb 22;159(4):375–380. doi: 10.1001/archinte.159.4.375. [DOI] [PubMed] [Google Scholar]
- 37.Del Rosso A, Alboni P, Brignole M, Menozzi C, Raviele A. Relation of clinical presentation of syncope to the age of patients. Am J Cardiol. 2005 Nov 15;96(10):1431–1435. doi: 10.1016/j.amjcard.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 38.Middlekauff HR, Stevenson WG, Stevenson LW, Saxon LA. Syncope in advanced heart failure: high risk of sudden death regardless of origin of syncope. J Am Coll Cardiol. 1993;21(1):110–116. doi: 10.1016/0735-1097(93)90724-f. [DOI] [PubMed] [Google Scholar]