Figure 5. Palmitoylation regulates tyrosine phosphorylation of NR2A and NR2B by the Src family tyrosine kinase Fyn.
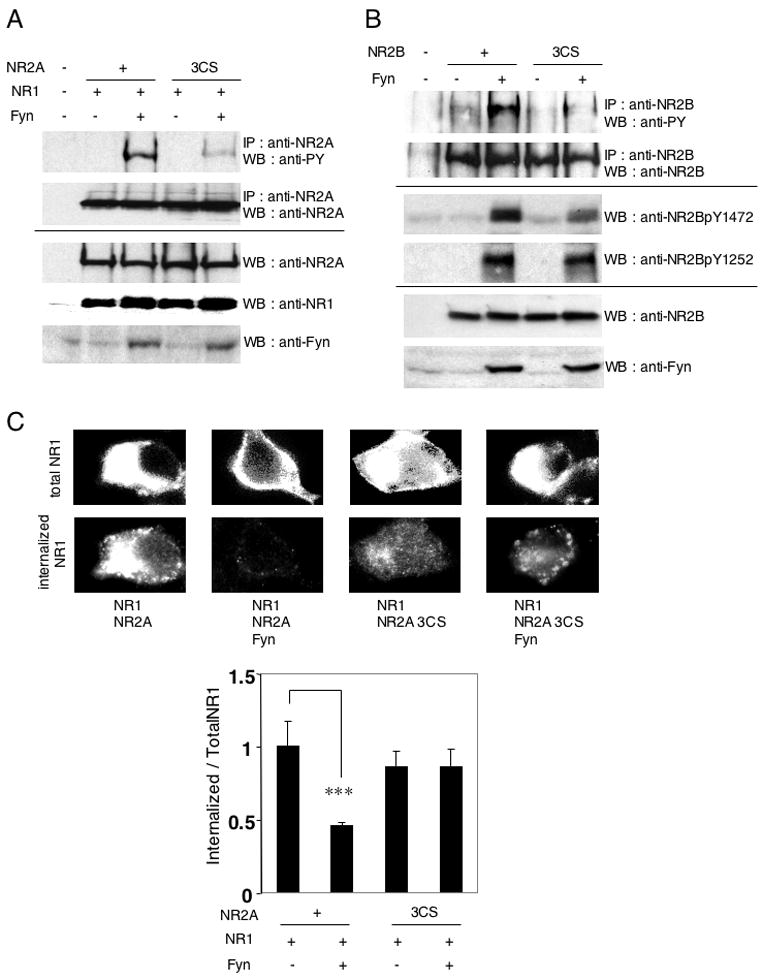
(A) Fyn-mediated tyrosine phosphorylation of NR2A in NR1/NR2A and NR1/NR2A 3CS receptors. HEK 293T cells were cotransfected with NR2A or NR2A 3CS and NR1 in the presence or absence of Fyn. Transfected cells were solubilized and then immunoprecipitated with anti-NR2A antibodies, followed by probing with phosphotyrosine antibody (PY20) (upper top panel). Immunoprecipitation (IP, upper bottom) and total protein expression (cell lysate) of each protein (lower panels) were confirmed by western blotting (WB). (B) Fyn-mediated tyrosine phosphorylation of NR2B and NR2B 3CS. The phosphotyrosine signals (PY20) were shown (upper top). IP of NR2B (top), site-specific tyrosine phosphorylation (NR2BpY1472 and NR2BpY1252, middle) and total protein expression (cell lysate) of each protein (bottom) were confirmed by WB. (C) Effect of Fyn on NMDA receptor endocytosis in transfected HEK 293T cells. Representative patterns of total and constitutive internalized NR1 were shown (top). The ratio of fluorescence intensities of internalized anti-NR1 antibodies to total NR1 expression in transfected HEK 293T cells (bottom, four bars (NR1/NR2A, NR1/NR2A/Fyn, NR1/NR2A 3CS, and NR1/NR2A 3CS/Fyn), n = 22, n = 28, n = 29, n = 34 cells, respectively, F = 5.276, p < 0.002, ANOVA). ***p < 0.001 compared with combination without Fyn. Error bars indicate sem.
