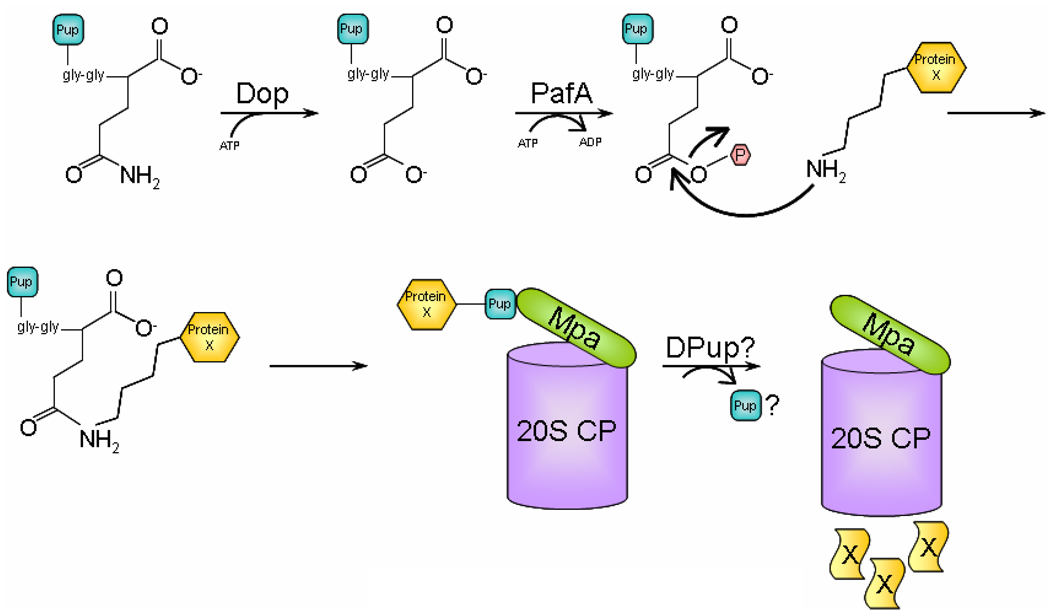
Fig. 1. Model for protein degradation by the Pup-proteasome system (PPS).
Tagging of a proteasome substrate, such as Protein X, is initiated with deamidation of Pup by Dop and ATP binding. Deamidated Pup then binds to PafA that with ATP hydrolysis catalyzes conjugation of a Pup monomer to Protein X. PafA facilitates the nucleophilic attack by an amino group present in Protein X to the γ-carboxylate of the C-terminal Glu residue in Pup. As a result, Protein X gets mono-pupylated; it is unknown if poly-Pup chains can form. Subsequently, Pup binds to Mpa, which might promote unfolding and translocation of Protein X into the 20S CP. During translocation, Pup might be removed by de-pupylating (DPUP) enzymes. Finally, Protein X is degraded to small peptides by the proteolytic activity in the central chamber of the 20S CP.