Table 1.
Vibrational frequencies (cm−1) of Fe/O2 species, the corresponding calculated 18O EIEs, and experimental 18O E/KIEs.
| molecule | Frequency (cm−1) |
18O EIE (calc)a | 18O EIE (expt)b | 18O KIE (expt)c | ||
|---|---|---|---|---|---|---|
| mode | ν16-16 | ν18-16 | ||||
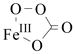
| FeIII-OO•− | Fe-Od | 555 | 526 | 1.0080 | 1.0054 (Mb) | NDe |
| O-Od | 1136 | 1100f | ||||
| FeIII-OOH | Fe-Og | 621 | 599 | 1.0172 | 1.0113 (Hr) | 1.0120 (HppE) |
| O-Og | 844 | 820f | ||||
| O-Hh | 3539 | 3527 | ||||
| O-O-Hh | 1205 | 1199 | ||||
| FeIII-OOtBu | Fe-Oi | 637 | 612 | 1.0187 | ND | ND |
| O-Oj | 860 | 829f | ||||
| O-tBuj | 746 | 738 | ||||
 |
Fe-Ok | 547 | 524 | 1.0143 | ND | 1.0102 (TauD) |
| O-Ok | 884 | 863f | ||||
| O-Ck | 965 | 946 | ||||
| O-C-Ok | 728 | 710 | ||||
| FeIV=O | Fe-Ol | 821 | 787 | 1.0287 | ND | 1.0215 (ACCO) |
The 18O EIEs were calculated using the Bigeleisen-Mayer equation and known frequencies (Ref 8).
Measured 18O EIEs for O2-binding proteins myoglobin (Mb) and hemerythrin (Hr) (Ref 14).
This work.
Ref 15.
Not determined.
ν18-16 was calculated as follows: ν18-16 = (ν16-16 ν 18-18)½ (Ref 8).
Ref 1.
Ref 14.
Ref 16.
Ref 17.
Ref 18.
Ref 7.
