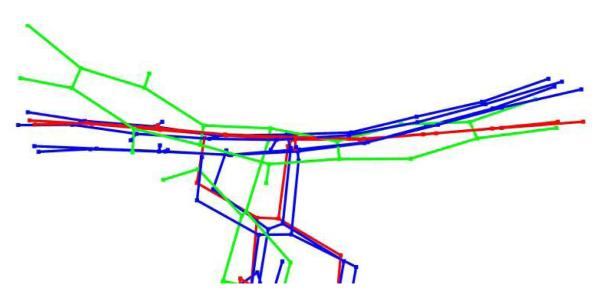
Figure 8.
Overlap of averaged atomic positions of reduced FAD in molecular dynamics simulations. Low energy conformations of reduced flavin are bent at the center ring of the isoalloxazine moiety. UDP-[3-F]Galf 4-flavin of conformation A, colored red, has the flattest isoalloxazine moiety, while UDP-Galf 1-flavin of conformation A, colored green, has the most bent structure. The difference is speculated to arise from unfavorable interactions of negative charges on 3-F of UDP-[3-F]Galf 4 with O4 of FAD as opposed to hydrogen bonding of the 3-OH” of UDP-Galf 1 with O4 of FAD. The FAD structures from both ligands in conformation B are shown in blue for comparison.