Figure 6.
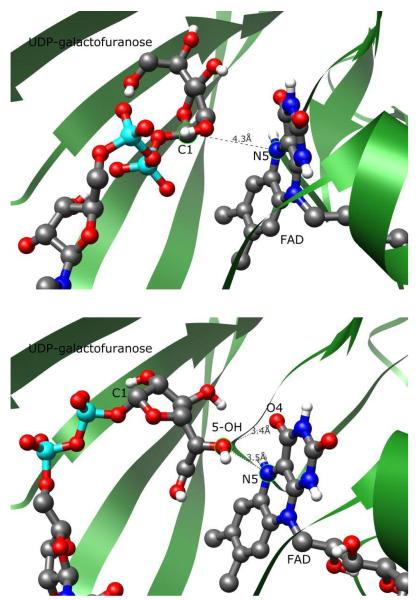
Contrast of UDP-Galf 1-flavin interactions in conformations A (above) and B (below). Conformation A positions the C1 carbon of the galactofuranose moiety 4.3 Å from N5, appropriately orientated for a nucleophilic attack. Conformation B moves the orientation of the galactofuranose ring perpendicular to the flavin plane, with C1 7.2 Å away from N5. The 5-OH of the galactose ring is closest to the flavin moiety.