Figure 3.
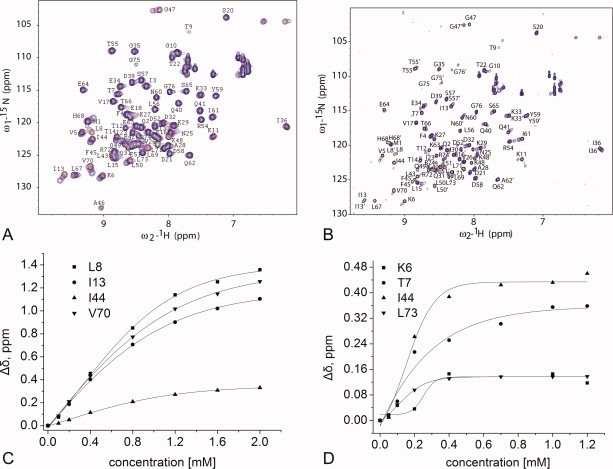
NMR analysis of the interaction of the hFAF1-UBA [1–81] with 15N mono- and di-Ub. Portions of 1H-15N HSQC spectra of 15N-mono-Ub (A) and 15N-di-Ub (B) in the presence of 2 equivalents of unlabeled the hFAF1-UBA [1–81] at 298 K. In (A), 0.2 mM 15N-mono-Ub with 0.1 mM hFAF1-UBA (magenta) and 0.4 mM hFAF1-UBA (blue). In (B), 0.2 mM 15N di-Ub with 0.1 mM hFAF1-UBA (magenta) and 0.4 mM hFAF1-UBA (blue). Plots of the chemical shift changes (Δδ = [(Δδ1H)2 + (Δδ15N/5)2]2) for the selected residues of ubiquitin on the addition of the hFAF1-UBA domain to the (C) mono-Ub and (D) di-Ub. The values of the dissociation constants (Kd) derived from these analyses are 1.47 ± 0.10 mM for mono-Ub and 0.10 ± 0.01 mM for di-Ub, respectively.
