Figure 4.
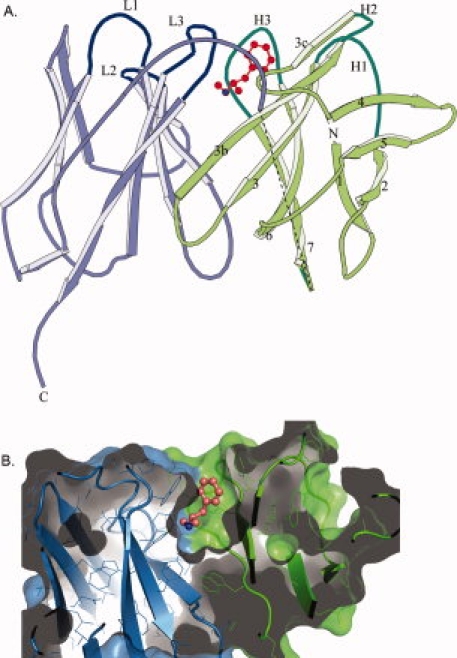
scFv6H4 METH binding. (A) A ribbon representation of scFv6H4:METH complex. The scFv6H4 consists of variable light chain domain (VL) (blue) and a variable heavy chain domain (VH) (green). Each domain is made up of two antiparallel pleated β-sheets. One sheet comprises four β-strands (marked as 1, 2, 4, and 5) and the other five strands (marked as 3c, 3b, 3, 6, and 7). The antigen binding site is formed by three CDR loops from the light chain (marked as L1, L2, and L3) and three CDR loops from the heavy chain (marked as H1, H2, and H3). The METH ligand is shown in red. The heavy and the light chains are joined together through a 15 amino acid peptide linker. Nine of these linker residues were not clearly visible in the electron density map of the METH complex and they are indicated by a dashed line in this figure. In the MDMA complex, only five residues are missing. The disordered residues are more than 20 Å away from the binding site. (B) A surface (solvent exclusion) representation of scFv6H4 bound to METH illustrating the deep binding pocket. The representation has been colored with the same colors used in (A). METH is shown as a ball and stick figure colored in salmon. The protein was clipped in the plane of the paper to show the interior of the binding pocket. Representation was rendered using PyMol (Delano Scientific, LLC).
