Figure 5.
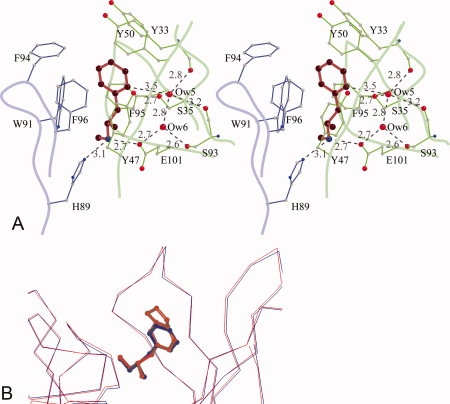
METH/MDMA binding pockets. (A) A stereoscopic view of methamphetamine binding pocket. Methamphetamine is shown in red, the light chain residues in blue, and the heavy chain residues in green. The aromatic ring of METH is surrounded by seven aromatic side chains. There is a salt bridge interaction between the cationic nitrogen and one of the carboxylate oxygen atoms of GluH101 from the heavy chain. The cationic nitrogen atom also forms a hydrogen bond with HisL89. There are two water molecules—Ow(5) and Ow(6) in the binding cavity. Ow(6) forms hydrogen bonds with Ow(5) and the side chains of GluH101 and SerH93. Ow(5), in addition, forms hydrogen bonds with the main chain carbonyl of TyrH33, SerH93, and the side chain of SerH35. Moreover, Ow(5) is positioned to form a C—H⋯O hydrogen bond with the aromatic ring of METH. The H⋯O distance is 2.6 Å and the C—H⋯O angle is 143°. (B) A comparison of METH and MDMA binding. The Cα-atoms of the scFv6H4:METH structure (blue) with scFv6H4:MDMA structure (red) are superposed (root mean square deviation of 0.23 Å for the Cα atoms). In the MDMA complex, the aromatic ring and the cationic nitrogen occupy the same locations retaining the same interactions.
