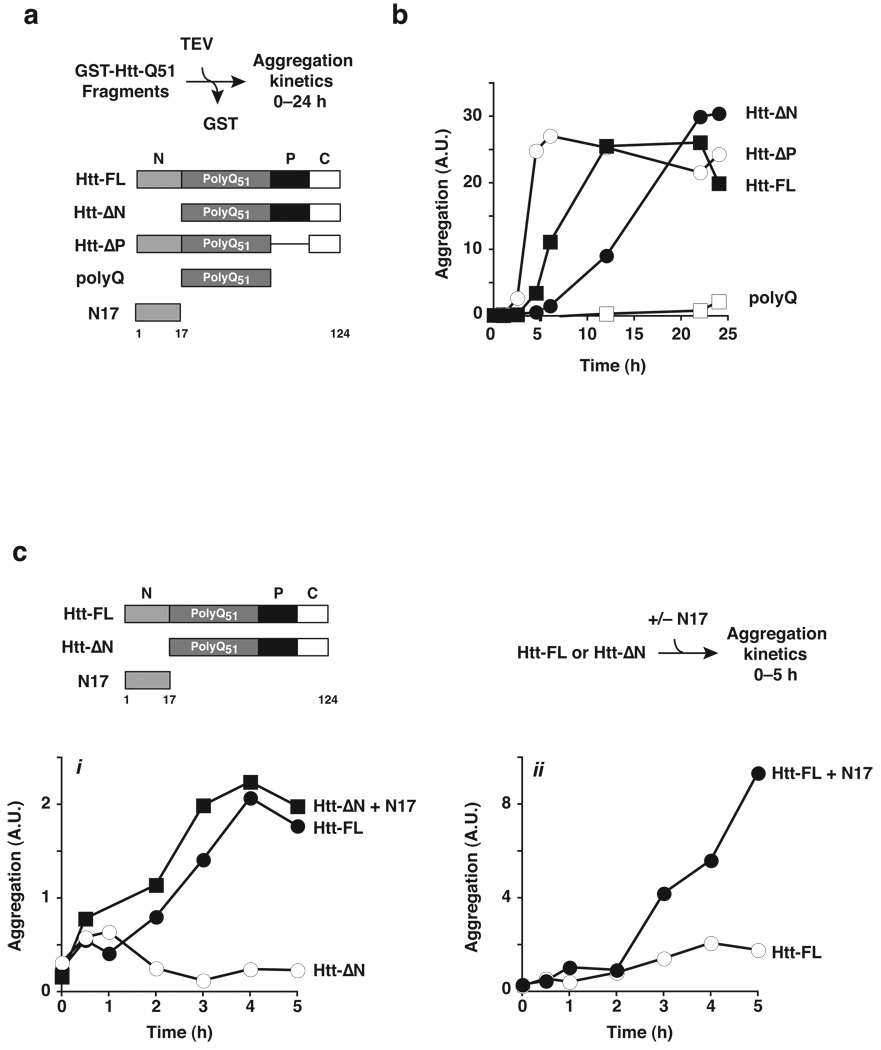
Figure 2. The N-terminus of huntingtin promotes rapid polyQ aggregation.
(a) Htt-Exon1 fragments used to analyze the contribution of individual domains towards aggregation. Formation of SDS-insoluble, heat-stable aggregates was determined by filter-trapping and quantified by infrared Li-Cor imaging as described in Online Methods19,24 (Supplementary Figs. 2 and 3). (b) Aggregation kinetics of Htt-exon1 fragments (Supplementary Fig. 4). Aggregation was initiated by TEV protease addition (time = 0 h) to GST- Htt-exon1 forms. All Htt variants were efficiently cleaved by TEV protease with similar kinetics (Supplementary Fig. 5). (c) Trans addition of N17 peptide enhances Htt-exon1 aggregation kinetics. A synthetic peptide containing 17 amino acids of the N-terminal region (N17Htt) was added to Htt-ΔN (i) or to full-length Htt-exon1 (ii) and assayed as in (b). Similar results were obtained when N17 was generated by TEV cleavage from a GST fusion (data not shown). Of note, CD and NMR indicate that N17 by itself was fully soluble at the concentrations used here (data not shown).