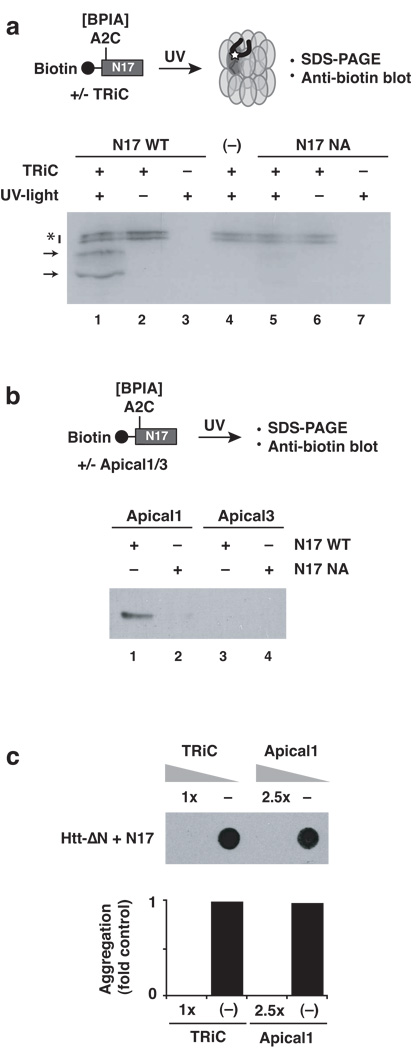
Figure 5. The hydrophobic surface of the N17 helix is the major Htt binding site for the chaperonin TRiC.
(a) Direct interaction of TRiC with N17 requires the hydrophobic face of the helix. Crosslinking to TRiC was tested for the wild type N17 peptide (N17-WT) or with a peptide carrying alanine substitutions in the hydrophobic side of the N17-helix (N17-NA). TRiC- and UV-dependent adducts were detected probing for the biotin moiety of the peptide. Non-specific background signals are labeled with an asterisk. (−): no peptide control. Results representative of at least three independent experiments are shown. (b) The substrate binding apical domain of TRiC subunit 1 mediates binding to the hydrophobic surface of N17. In vitro crosslinking using N17-WT indicate an interaction with the isolated apical domain of TRiC subunit 1. The interaction is based on similar contacts with intact TRiC, as crosslinking was perturbed by the NA mutation within N17. No interaction is observed to the apical domain of TRiC subunit 3. As with other TRiC substrates, specific subsets of TRiC subunits interact with different substrates. (c) Purified TRiC and Apical 1 domain neutralize the aggregation promoting effect of trans- addition of N17 to Htt-ΔN. (−): Ovalbumin non-specific negative control. The lower panel shows the quantification of signal observed on the membrane (upper panel).