Abstract
Purpose
The aim of this study was to determine if the size of zona pellucida thinning area by laser assisted hatching could affect the rates of pregnancy and implantation for vitrified-warmed embryo transfers at the cleavage-stage.
Methods
A total of 120 vitrified-warmed cleavage-stage embryo transfers were randomly assigned to either quarter or half of zona pellucida thinning group.
Results
The rates of clinical pregnancy (46.7 versus 25.0%) and implantation (32.0 versus 16.2%) were significantly greater in the half thinning group than in the quarter thinning group (P = 0.0218 and P = 0.0090, respectively).
Conclusions
The results of this investigation show that, in vitrified-warmed embryo transfers at the cleavage-stage, the size of zona pellucida thinning area by laser assisted hatching impacts the rate of clinical pregnancy and implantation and that half of zona pellucida thinning significantly increases both of these results compared with quarter of zona pellucida thinning.
Keywords: Cleavage-stage embryo transfer, Laser assisted hatching, Vitrification, Zona pellucida thinning
Introduction
Assisted reproductive technology generally produces surplus embryos that can be cryopreserved for later use. The success of cryopreservation programs will undoubtedly increase the cumulative pregnancy rates attained in IVF. Success associated with transfer of cryopreserved embryos has been generally lower than that obtained with fresh embryo transfer [1]. One explanation may be due to zona pellucida hardening during the freezing and thawing process. Therefore, artificial thinning or opening of the zona pellucida, i.e. assisted hatching, may be useful for frozen-thawed embryo transfer. However, there are only a few randomized studies focused on the benefit of assisted hatching following frozen-thawed embryo transfer that have reported controversial results with decreased [2], similar [3–5] or increased [6–9] implantation rates in the assisted hatching group compared with the control group. This is likely related to different assisted hatching techniques (chemical, enzyme or laser) and different methods of zona pellucida manipulation (thinning or opening), in addition to differences in the study design, patient characteristics, and selection criteria. Assisted hatching by 1.48 μm diode laser appears to be associated with a better outcome than that after chemical [10] or mechanical [11] methods and has enables a reproducible equally sized zona pellucida thinning or opening. Mantoudis et al., in a retrospective study, found a higher pregnancy rate after quarter of zona pellucida thinning than after ∼8–10 μm opening or thinning by laser assisted hatching [12]. Similarly, Ng et al., in a double-blind randomized study, found higher ongoing pregnancy and implantation rates after more than quarter of zona pellucida thinning by laser assisted hatching than after 30 μm opening in frozen-thawed embryo transfers [13]. In addition, in our previous retrospective study, quarter of zona pellucida thinning by laser assisted hatching significantly increased the rates of pregnancy and implantation in frozen-thawed embryo transfers at the cleavage-stage, compared with controls without assisted hatching (unpublished data). On the other hand, we have recently shown in a retrospective study that half of zona pellucida opening by laser assisted hatching improves pregnancy, implantation and delivery rates of frozen-thawed cleavage-stage embryos that were cultured to blastocyst after thawing and assisted hatching was conducted at the blastocyst-stage, compared with 40 μm (about one eighth of zona pellucida) opening [14]. It is possible that half of zona pellucida thinning in cryopreserved embryo transfer may be associated with higher pregnancy rate than is quarter thinning. However, no randomized studies exist comparing quarter of zona pellucida thinning with half thinning. The aim of this randomized prospective study was to assess whether half of zona pellucida thinning by laser assisted hatching could enhance the clinical outcome of vitrified-warmed embryo transfers at the cleavage-stage compared with quarter of zona pellucida thinning.
Materials and methods
Patients
Infertile patients who were attending at Kinutani Women’s Clinic were recruited if they had at least one vitrified embryo available for transfer. The study was confined to non-donor and first vitrified-warmed embryo transfers. Fresh IVF attempts were conducted between 2004 and 2008. Embryos were generated by ICSI, by conventional IVF (cIVF) or by split cycle (ICSI and cIVF in combination), because it has been shown that no adverse effect was demonstrated concerning the implantation ability of such frozen-thawed embryos derived from by either ICSI or by cIVF [15–17]. The indications for IVF included tubal, endometriosis, male and others (mixed factors or unexplained infertilities). On the day of vitrified-warmed embryo transfer, patients were randomized by a laboratory technician according to a computer-generated randomization list in sealed envelopes into the quarter of zona pellucida thinning group or the half of zona pellucida thinning group. Both patients and clinicians were unaware of the group assignments until the completion of the study.
We do not have Institutional Review Board in our private clinic. Drs K. Kinutani, M. Kinutani, S. Okano and T. Kusuda are members of Japan Society of Obstetrics and Gynecology (JSOG) and Kinutani Women’s Clinic have been registered as certified fertility centres by JSOG. This study was conducted according to the guidelines of JSOG. Furthermore, each couple included in this study was asked to sign an approval consent form before enrolling in the study.
IVF procedure
Women were treated with gonadotrophin-releasing hormone (GnRH) analogue buserelin acetate (Mochida, Tokyo, Japan) from either the preceding mid-luteal phase in a long treatment protocol or second day of the cycle in a short treatment protocol. Ovarian stimulation was carried out with human menopausal gonadotrophin (Nikken, Tokyo, Japan) or urinary FSH (Fertinorm; Serono, Japan). Follicular development was monitored with serial (every third day) vaginal ultrasound examinations and serum oestradiol measurements. Human chorionic gonadotrophin (HCG; Teizo, Tokyo, Japan) was administered to women when dominant follicles reached a diameter of 18 mm. Oocytes were collected 35 h after HCG administration using a vaginal ultrasound-guided procedure and were incubated in human tubal fluid (HTF) medium (Irvine Scientific, CA, USA) containing 10% (v/v) serum substitute supplement (SSS; Irvine). Sperm preparation was carried out using discontinuous ISolate™ (Irvine) gradient. Mature oocytes were either inseminated with spermatozoa 5–7 h after oocyte retrieval at a concentration of 100,000 to 200,000 motile spermatozoa/ml for 5 to 10 oocytes (cIVF) or microinjected with a single spermatozoon (ICSI). The day of oocyte retrieval was considered as day 0. Fertilization was confirmed at 24–25 h after oocyte retrieval (day 1) by the presence of two pronuclei.
Fertilized oocytes were transferred and cultured in P-1 (Irvine) containing 10% (v/v) SSS (Irvine) from day 1 to day 3. All oocytes and embryos were incubated at 37°C in an atmosphere of 6% CO2, 5% O2 and 89% N2. Fresh embryos were transferred on day 2 or day 3. The day of embryo transfer and vitrified were determined according to the clinical or patient’s schedules. In our previous clinical experience, the rates of pregnancy and implantation after fresh or cryopreserved day 2 and day 3 transfers were similar. Therefore, in the present study, the results of vitrified-warmed day 2 and day 3 transfers were analyzed together. Some patients abandoned fresh embryo transfer in order to avoid potential risks of ovarian hyperstimulation syndrome. Good quality embryos were defined as those having regular blastomeres, <20% fragments and no multinucleated blastomeres and those containing at least three cells on day 2 or six cells on day 3. Embryos that in addition had cleaved early were considered top quality embryos, and were first choice for fresh transfer. After the transfer of fresh embryos, all surplus embryos were vitrified on day 2 or day 3 only for patients who had at least one good quality embryo in their surplus embryos.
Vitrification of embryo
The embryos were vitrified by the method developed by Kuwayama et al. [18] using a cryotop (Kitazato Supply Co., Fujinomiya, Japan), albeit with slight modifications, and has been described previously [19, 20]. The cryotop consists of a 0.4 mm wide × 20 mm long × 0.1 mm thick polypropylene strips attached to a plastic handle and equipped with a cover straw. One embryo was loaded for each cryotop. As the base medium, modified HTF medium-HEPES (Irvine) plus 20% (v/v) SSS (Irvine) was used. The equilibration solution contained 7.5% (v/v) ethylene glycol (Sigma Chemical Co., MO, USA) and 7.5% (v/v) dimethyl sulphoxide (Kanto Chemical Co., Tokyo, Japan). The vitrification solution was composed of 15% (v/v) ethylene glycol, 15% (v/v) dimethyl sulphoxide and 0.5 mol/l sucrose (Nacalai Tesque, Inc., Kyoto, Japan). The embryo was incubated in 1 ml of 30°C equilibration solution for 10 min, confirming shrinkage and re-expansion before exposure to the vitrification solution. After equilibration, the embryo was then incubated in 1 ml of 30°C vitrification solution and loaded, within 45 s, onto the tip of the cryotop with ∼1 μl of cryoprotectant solution. Then the cryotop was immediately submerged into liquid nitrogen which had been filter sterilized through a 0.22 μm filter (Millipore, Cork, Ireland) [21] and under the liquid nitrogen the plastic cover was placed over the strip to provide protection during storage.
Warming of embryos
Vitrified embryos considered top quality in each patient’s vitrified embryos were warmed on the day of embryo transfer. The warming procedure was done as follows. The protective cover was removed in liquid nitrogen and the end of the polypropylene strip was immersed directly into 1 ml of 37°C 1.0 mol/l sucrose solution for 1 min. The embryos were then transferred into 1 ml of 37°C 0.5 mol/l sucrose solution for 3 min and washed twice in the base medium for 5 min.
Assisted hatching
As soon as warming of embryos was completed, assisted hatching was performed by the method as described previously, albeit with slight modifications [14, 22]. Warmed embryos were placed under mineral oil within a 50 μl microdroplet of 37°C Sperm Washing Medium (Irvine) in a Petri dish and positioned on the phase-contrast inverted microscope stage. Before assisted hatching, the thickness of zona pellucida was measured at the 3, 6, 9 and 12 o’clock positions from the inside to the outside, and the average thickness was calculated.
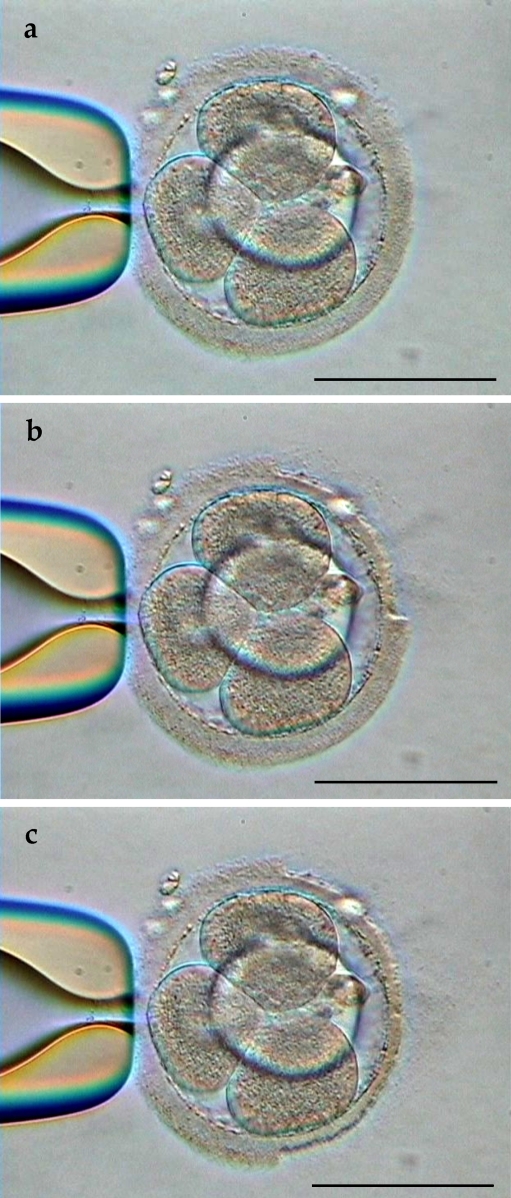
Quarter or half of zona pellucida thinning using laser (Zilos-tk Laser, Hamilton Thorne Research, Beverly, MA, USA) was performed as follows. Embryos were stabilized with a holding pipette held at the 9 o’clock position (Fig. 1a), and positioned with the laser target located on the outer edge of the zona pellucida. The power of laser was 100% and the pulse duration was 300 μs. By this setting, a 5 μm hole was formed in the zona pellucida by one laser shot. Quarter or half of zona pellucida thinning was performed by thinning the zona pellucida at a depth of 50–80% of the zona pellucida thickness, estimated from an area of empty perivitelline space, initiated at one point and continued until quarter or half of the zona pellucida was irradiated, i.e. laser thinning was initiated at the 12 o’clock position and consecutive irradiations were generated until the 3 o’clock position (quarter thinning) (Fig. 1b) or the 6 o’clock position (half thinning) (Fig. 1c) was reached. It took about 1.5 min to complete quarter thinning procedure and about 3.0 min to complete half thinning procedure per embryo. After the assisted hatching procedure was completed, the embryos were rinsed several times and were cultured in P-1 (Irvine) containing 10% (v/v) SSS (Irvine). The interval between warming and embryo transfer was 3 h.
Fig. 1.
A vitrified-warmed human cleavage-stage embryo a stabilized with a holding pipette before assisted hatching b after quarter of zona pellucida thinning by laser assisted hatching c after half of zona pellucida thinning by laser assisted hatching. Bar represents 100 μm
Assessment of survival of warmed embryo
The post warming survival of embryos was observed 1–2 h after warming under a microscope. Embryos were classified as fully intact (100% cells morphologically intact), partially damaged (≥50% cells morphologically intact) or degenerated (<50% cells morphologically intact). The survival rate was defined as the number of warmed embryos with ≥50% cells morphologically intact over the total number of warmed embryos × 100. Vitrified-warmed embryo transfers were performed using only embryos with ≥50% morphologically intact blastomeres.
Endometrial preparation
Vitrified-warmed embryo transfer was performed in hormonal replacement treatment cycles. All women received transdermal oestradiol (Estraderm 1.4–5.8 mg, Kissei, Tokyo, Japan) with gonadotrophin-releasing hormone analogue for the preparation of the endometrium. The administration of progesterone (vaginal 400 mg daily) was initiated when endometrial thickness exceeded 10 mm. Vitrified-warmed day 2 and day 3 embryo transfers were scheduled on day 2 and on day 3 after the initiation of progesterone treatment respectively. One to two embryos were transferred into the patient’s uterus.
Assessment of pregnancy
In both fresh embryo transfer and vitrified-warmed embryo transfer outcomes, a clinical pregnancy was assessed by a positive fetal heartbeat on transvaginal ultrasound at 5–6 weeks of pregnancy. The implantation rate was calculated as the number of gestational sacs identified on transvaginal ultrasound per number of fresh or vitrified-warmed transferred embryos.
Statistical analysis
In our small pilot study, the pregnancy rate of vitrified-warmed embryo transfers at the cleavage-stage after quarter and half of zona pellucida thinning by laser assisted hatching was 27% and 54% respectively (unpublished data). The sample size required would be 118 patients to achieve statistical significance (P < 0.05) with a power of 0.8. The Mann-Whitney test, unpaired Student’s t test, chi-squared test and Fisher’s exact test were used as appropriate to determine statistical differences between groups. A P value of <0.05 was considered significant.
Results
Between February 2005 and September 2008, 167 couples were asked to participate in the study, but 45 couples declined to participate in the study and 2 were excluded because no embryos survived. Therefore, a total of 120 couples were recruited, and all completed the study.
Table 1 summarizes the patients’ demographic characteristics and IVF outcome. There were no differences between the quarter thinning and half thinning groups in the age of women at vitrification, primary infertility, secondary infertility, cause of infertility, the number of IVF attempts, the number of oocytes, the number of metaphase II oocytes, the percentage of cIVF or ICSI or split cycle and the number of embryos. The number of embryos transferred, clinical pregnancy rate, implantation rate, twin pregnancy rate, delivery rate, the number of embryos vitrified, the distribution between day 2 and day 3 embryos vitrified and the distribution between good quality day 2 and day 3 embryos vitrified after fresh embryo transfer were also comparable between the two groups.
Table 1.
Demographic characteristics and IVF outcomes between the quarter of zona pellucida thinning and the half of zona pellucida thinning groups
| Parameter | quarter (n = 60) | half (n = 60) |
|---|---|---|
| Mean age of women at vitrification (years) ± SD | 34.0 ± 4.4 | 34.4 ± 4.3 |
| No. with primary/secondary infertility | 42/18 | 48/12 |
| Cause of infertility | ||
| Tuboperitoneal (%) | 13 (21.7) | 8 (13.3) |
| Endometriosis (%) | 2 (3.3) | 1 (1.7) |
| Male (%) | 9 (15.0) | 11 (18.3) |
| Others (%) | 36 (60.0) | 40 (66.7) |
| Mean no. of IVF attempts ± SD | 1.4 ± 1.2 | 1.3 ± 0.6 |
| Mean no. of oocytes ± SD | 11.9 ± 7.2 | 11.4 ± 7.6 |
| Mean no. of MII oocytes ± SD | 8.9 ± 5.1 | 9.0 ± 6.0 |
| Insemination method | ||
| Conventional IVF (%) | 5 (8.3) | 4 (6.7) |
| ICSI (%) | 34 (56.7) | 33 (55.0) |
| Split cycle (%) | 21 (35.0) | 23 (38.3) |
| Mean no. of embryos ± SD | 6.7 ± 4.0 | 6.8 ± 4.8 |
| No. of fresh embryo transfer cycles | 46 | 46 |
| Mean no. of embryos transferred ± SD | 2.0 ± 0.2 | 1.9 ± 0.4 |
| No. of fresh embryos transferred | 90 | 88 |
| No. of clinical pregnancies (%) | 16 (34.8) | 14 (30.4) |
| No. of embryos implanted (%) | 19 (21.1) | 16 (18.2) |
| No. of twin pregnancies (%) | 2 (12.5) | 0 (0) |
| No. of deliveries (%) | 12 (26.1) | 11 (23.9) |
| Mean no. of embryos vitrified ± SD | 5.1 ± 4.4 | 5.2 ± 5.0 |
| No. of embryos vitrified | 308 | 307 |
| No. of day 2/day 3 embryos vitrified | 157/151 | 150/157 |
| No. of good quality embryos vitrified | 124 | 120 |
| No. of good quality day 2/day 3 embryos vitrified | 57/67 | 58/62 |
There were no statistically significant between the two groups. MII metaphase II; ICSI intracytoplasmic sperm injection; Split cycle = ICSI and conventional IVF in combination. Good quality embryos were defined as those having regular blastomeres, <20% fragments and no multinucleated blastomeres and those containing at least three cells on day 2 or six cells on day 3
Table 2 summarizes the outcomes of vitrified-warmed embryo transfer. The distribution between day 2 and day 3 embryos warmed, the distribution between good quality day 2 and day 3 embryos warmed, survival rate, the number of embryos transferred and the thickness of zona pellucida were similar between the two groups. On the other hand, clinical pregnancy and implantation rates were significantly higher in the half thinning group compared with the quarter thinning group (46.7% versus 25.0%, P = 0.0218; 32.0% versus 16.2%, P = 0.0090). No differences in the twin pregnancy and miscarriage rates per clinical pregnancy were found between the two groups.
Table 2.
Comparison of outcomes of vitrified-warmed cleavage-stage embryo transfers between the quarter of zona pellucida thinning and the half of zona pellucida thinning groups
| Parameter | quarter (n = 60) | half (n = 60) | P-value |
|---|---|---|---|
| No. of embryos warmed | 104 | 107 | – |
| No. of day 2/day 3 embryos warmed | 48/56 | 54/53 | NS |
| No. of good quality embryos warmed | 65 | 61 | NS |
| No. of good quality day 2/day 3 embryos warmed | 28/37 | 27/34 | NS |
| No. of embryos survived (%) | 99 (95.2) | 103 (96.3) | NS |
| Survival classification | |||
| Fully intact (%) | 95 (91.3) | 97 (90.7) | NS |
| Partially damaged (%) | 4 (3.9) | 6 (5.6) | NS |
| Degenerated (%) | 5 (4.8) | 4 (3.7) | NS |
| Mean no. of embryos transferred ± SD | 1.7 ± 0.5 | 1.7 ± 0.6 | NS |
| Mean zona pellucida thickness ± SD (μm) | 18.7 ± 0.5 | 18.8 ± 0.6 | NS |
| No. of clinical pregnancies (%) | 15 (25.0) | 28 (46.7) | 0.0218 |
| No. of embryos implanted (%) | 16 (16.2) | 33 (32.0) | 0.0090 |
| No. of twin pregnancies (%) | 1 (6.7) | 5 (17.9) | NS |
| No. of miscarriages (%) | 2 (13.3) | 3 (10.7) | NS |
NS not significant. Good quality embryos were defined as those having regular blastomeres, <20% fragments and no multinucleated blastomeres and those containing at least three cells on day 2 or six cells on day 3. Survival classification: Fully intact = 100% cells morphologically intact, Partially damaged = ≥50% cells morphologically intact, Degenerated = <50% cells morphologically intact
Discussion
This study indicates that half of zona pellucida thinning by laser assisted hatching significantly improves the pregnancy and implantation rates of vitrified-warmed embryo transfers at the cleavage-stage compared with quarter of zona pellucida thinning.
We have recently shown in a retrospective study that half of zona pellucida opening improves the pregnancy, implantation and delivery rates of frozen-thawed cleavage-stage embryos that were cultured to blastocyst after thawing and assisted hatching was conducted at the blastocyst-stage, compared with 40 μm (about one eighth of the zona pellucida) opening [14]. In addition, the results of half of zona pellucida thinning demonstrated significantly higher pregnancy and implantation rates compared with quarter thinning in the present study. Therefore, our results suggest that the area of zona pellucida thinning or opening can affect the outcome of cryopreserved embryo transfer and that higher pregnancy and implantation rates can be attained as the area of zona pellucida thinning or opening increases up to half. However, Yano et al. reported higher clinical pregnancy rate (32.2%) after the transfer of partial zona pellucida thinning embryos using acid solution compared with circumferential zona pellucida thinning embryos (27.4%) [23]. Further study evaluating if more than half of zona pellucida thinning could increase the clinical outcome of cryopreserved embryo transfers compared with half thinning may be needed.
Significantly higher implantation rate (32.0%) was observed after the transfer of half of zona pellucida thinning vitrified-warmed cleavage-stage human embryos compared with that of after quarter of zona pellucida thinning embryos (16.2%) in the present study. This might be due to the difference of hatching rates under these treatments. In our previous animal study, the rate of observable progressing completely hatched blastocyst of vitrified-warmed eight-cell mouse embryos after half of zona pellucida thinning by laser assisted hatching at the time of warming significantly enhanced (90.0%; 72/80) compared with that of after quarter of zona pellucida thinning (76.3%; 61/80) (unpublished data). Therefore, it is suggested that quarter of zona pellucida thinning of vitrified-warmed human embryo transfer at the cleavage-stage is inadequate for the completion of hatching process in some cases, however, it can be considerably improved by half of zona pellucida thinning.
It is difficult to achieve half of zona pellucida thinning with the use of an acid solution and the chemical needs to be washed out to prevent any damage to the embryo. The laser offers many advantages over traditional methods in a clinical situation where half of zona pellucida needs to be thinned. However, this procedure may pose potential harm to embryos by heat shock. Hartshorn et al. demonstrated that mouse embryos at the eight-cell stage are able to respond to thermal shock by activating heat shock protein (hsp) production, as shown by the sharp increase in hsp70i transcription that follows embryo exposure to elevated temperature [24]. They also reported that in eight-cell mouse embryos, the procedure of a whole of zona pellucida opening with laser did not stimulate hsp70i expression in all blastomeres [25]. In addition, our results for half of zona pellucida thinning group by laser demonstrated higher pregnancy and implantation rates without increasing the miscarriage rate compared with quarter thinning group. Moreover, Kanyo and Konc reported that there was no evidence of an increase in chromosomal aberrations or congenital malformations for 134 children born after laser assisted hatching [26]. However, the fact that a pregnancy is established does not preclude that there are other underlying anomalies. Therefore, further long-term studies, such as looking at the birth defects and other anomalies that can occur later, are needed to confirm and assess the safety of half of zona pellucida thinning by laser assisted hatching.
Four very recent, well-designed prospective studies evaluating the effectiveness of quarter or more than quarter of zona pellucida thinning using laser for frozen-thawed cleavage-stage embryo transfers reported controversial results compared with the control group with no assisted hatching [3, 5, 7, 8]. Ng et al. reported no improvement in pregnancy and implantation rates after the transfer of more than quarter of zona pellucida thinned frozen-thawed embryos (12.5% and 9.0%, respectively) compared with control embryos (15% and 6.8%, respectively) [3]. This result was very similar to that obtained by the report of Petersen et al. [5]. In this study, no benefit of quarter of zona pellucida thinning by laser was demonstrated in frozen-thawed embryo transfers. In contrast, the study by Ge et al. showed that quarter of zona pellucida thinning using laser increased the pregnancy and implantation rates of frozen-thawed embryo transfers [8]. The pregnancy and implantation rates in assisted hatching group were 25.0% and 17.7% while those in the control group were 14.0% and 7.3% respectively. Balaban et al. also showed that quarter of zona pellucida thinning using laser significantly increased the pregnancy and implantation rates (40.9% and 20.1%, respectively) compared with control (27.3% and 9.9%, respectively) [7]. In this study, assisted hatching was performed about 20 h after thawing and only on embryos that showed evidence of cleavage. On the other hand, the present study indicated that half of zona pellucida thinning using laser before vitrified-warmed embryo transfer could significantly improve the rates of pregnancy and implantation compared with quarter thinning. Furthermore, in our previous study, the resistance to enzymatic removal of zona pellucida of vitrified-warmed blastocysts (120.4 ± 10.2s) [27] significantly increased compared with blastocysts developed from frozen-thawed cleavage-stage embryos (104.9 ± 10.1s) [14]. These observations suggest that more zona pellucida hardening can occur after vitrification process compared with slow-freezing process. Therefore, the outcomes of the study evaluating the effectiveness of laser assisted hatching for cryopreserved embryo transfer may be different with the cryopreservation methods or with the area of zona pellucida thinning.
Conclusions
In vitrified-warmed cleavage-stage embryo transfers, half of zona pellucida thinning by laser assisted hatching could significantly increase the rates of clinical pregnancy and implantation compared with quarter of zona pellucida thinning. If the safety of laser assisted hatching is confirmed, half of zona pellucida thinning by laser assisted hatching should be performed routinely in vitrified-warmed embryo transfers at the cleavage-stage.
Footnotes
Capsule
The size of the zona pellucida thinning area by laser assisted hatching has impact on the outcomes of vitrified-warmed embryo transfers at the cleavage-stage.
References
- 1.Andersen AN, Goossens V, Ferraretti AP, Bhattacharya S, Felberbaum R, de Mouzon J, et al. Assisted reproductive technology in Europe, 2004: results generated from European registers by ESHRE. Hum Reprod. 2008;23:756–71. [DOI] [PubMed]
- 2.Primi MP, Senn A, Montag M, Van der Ven H, Mandelbaum J, Veiga A, et al. A European multicentre prospective randomized study to assess the use of assisted hatching with a diode laser and the benefit of an immunosuppressive/antibiotic treatment in different patient populations. Hum Reprod. 2004;19:2325–33. [DOI] [PubMed]
- 3.Ng EH, Naveed F, Lau EY, Yeung WS, Chan CC, Tang OS, et al. A randomized double-blind controlled study of the efficacy of laser-assisted hatching on implantation and pregnancy rates of frozen-thawed embryo transfer at the cleavage stage. Hum Reprod. 2005;20:979–85. [DOI] [PubMed]
- 4.Sifer C, Sellami A, Poncelet C, Kulski P, Martin-Pont B, Bottero J, et al. A prospective randomized study to assess the benefit of partial zona pellucida digestion before frozen-thawed embryo transfers. Hum Reprod. 2006;21:2384–9. [DOI] [PubMed]
- 5.Petersen CG, Mauri AL, Baruffi RL, Oliveira JB, Felipe V, Massaro FC, et al. Laser-assisted hatching of cryopreserved-thawed embryos by thinning one quarter of the zona. Reprod Biomed Online. 2006;13:668–75. [DOI] [PubMed]
- 6.Gabrielsen A, Agerholm I, Toft B, Hald F, Petersen K, Aagaard J, et al. Assisted hatching improves implantation rates on cryopreserved-thawed embryos. A randomized prospective study. Hum Reprod. 2004;19:2258–62. [DOI] [PubMed]
- 7.Balaban B, Urman B, Yakin K, Isiklar A. Laser-assisted hatching increases pregnancy and implantation rates in cryopreserved embryos that were allowed to cleave in vitro after thawing: a prospective randomized study. Hum Reprod. 2006;21:2136–40. [DOI] [PubMed]
- 8.Ge HS, Zhou W, Zhang W, Lin JJ. Impact of assisted hatching on fresh and frozen-thawed embryo transfer cycles: a prospective, randomized study. Reprod Biomed Online. 2008;16:589–96. [DOI] [PubMed]
- 9.Valojerdi MR, Eftekhari-Yazdi P, Karimian L, Ashtiani SK. Effect of laser zona pellucida opening on clinical outcome of assisted reproduction technology in patients with advanced female age, recurrent implantation failure, or frozen-thawed embryos. Fertil Steril. 2008;90:84–91. [DOI] [PubMed]
- 10.Hsieh YY, Huang CC, Cheng TC, Chang CC, Tsai HD, Lee MS. Laser-assisted hatching of embryos is better than the chemical method for enhancing the pregnancy rate in women with advanced age. Fertil Steril. 2002;78:179–82. [DOI] [PubMed]
- 11.Makrakis E, Angeli I, Agapitou K, Pappas K, Dafereras A, Pantos K. Laser versus mechanical assisted hatching: a prospective study of clinical outcomes. Fertil Steril. 2006;86:1596–600. [DOI] [PubMed]
- 12.Mantoudis E, Podsiadly BT, Gorgy A, Venkat G, Craft IL. A comparison between quarter, partial and total laser assisted hatching in selected infertility patients. Hum Reprod. 2001;16:2182–6. [DOI] [PubMed]
- 13.Ng EH, Lau EY, Yeung WS, Cheung TM, Tang OS, Ho PC. Randomized double-blind comparison of laser zona pellucida thinning and breaching in frozen-thawed embryo transfer at the cleavage stage. Fertil Steril. 2008;89:1147–53. [DOI] [PubMed]
- 14.Hiraoka K, Fuchiwaki M, Hiraoka K, Horiuchi T, Murakami T, Kinutani M, et al. Effect of the size of zona pellucida opening by laser assisted hatching on clinical outcome of frozen cleaved embryos that were cultured to blastocyst after thawing in women with multiple implantation failures of embryo transfer: a retrospective study. J Assist Reprod Genet. 2008;25:129–35. [DOI] [PMC free article] [PubMed]
- 15.Kowalik A, Palermo GD, Barmat L, Veeck L, Rimarachin J, Rosenwaks Z. Comparison of clinical outcome after cryopreservation of embryos obtained from intracytoplasmic sperm injection and in-vitro fertilization. Hum Reprod. 1998;13:2848–51. [DOI] [PubMed]
- 16.Aytoz A, Van den Abbeel E, Bonduelle M, Camus M, Joris H, Van Steirteghem A, et al. Obstetric outcome of pregnancies after the transfer of cryopreserved and fresh embryos obtained by conventional in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1999;14:2619–24. [DOI] [PubMed]
- 17.Hu Y, Maxson WS, Hoffman DI, Ory SJ, Eager S. A comparison of post-thaw results between cryopreserved embryos derived from intracytoplasmic sperm injection and those from conventional IVF. Fertil Steril. 1999;72:1045–8. [DOI] [PubMed]
- 18.Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11:608–14. [DOI] [PubMed]
- 19.Hiraoka K, Hiraoka K, Kinutani M, Kinutani K. Blastocoele collapse by micropipetting prior to vitrification gives excellent survival and pregnancy outcomes for human day 5 and 6 expanded blastocysts. Hum Reprod. 2004;19:2884–8. [DOI] [PubMed]
- 20.Hiraoka K, Fujimoto Y, Tateaki Y, Hiraoka K, Horiuchi T, Okano S, et al. Case report: two successful pregnancies following the transfer of re-vitrified human day 7 blastocysts developed from vitrified cleaved embryos. J Assist Reprod Genet. 2008;25:503–9. [DOI] [PMC free article] [PubMed]
- 21.Vajta G, Lewis IM, Kuwayama M, Greve T, Callesen H. Sterile application of the open pulled straw (OPS) vitrification method. Cryo Lett. 1998;19:389–92.
- 22.Hiraoka K, Fuchiwaki M, Hiraoka K, Horiuchi T, Murakami T, Kinutani M, et al. Zona pellucida removal and vitrified blastocyst transfer outcome: a preliminary study. Reprod Biomed Online. 2007;15:68–75. [DOI] [PubMed]
- 23.Yano K, Yano C, Kubo T, Ohashi I, Maeda N, Fukaya T. Chemical zona pellucida thinning with acidified Tyrode’s solution: comparison between partial and circumferential techniques. J Assist Reprod Genet. 2007;24:471–5. [DOI] [PMC free article] [PubMed]
- 24.Hartshorn C, Anshelevich A, Wangh LJ. Rapid, single-tube method for quantitative preparation and analysis of RNA and DNA in samples as small as one cell. BMC Biotechnol. 2005;5:2. [DOI] [PMC free article] [PubMed]
- 25.Hartshorn C, Anshelevich A, Wangh LJ. Laser zona drilling does not induce hsp70i transcription in blastomeres of eight-cell mouse embryos. Fertil Steril. 2005;84:1547–50. [DOI] [PubMed]
- 26.Kanyo K, Konc J. A follow-up study of children born after diode laser assisted hatching. Eur J Obstet Gynecol Reprod Biol. 2003;110:176–80. [DOI] [PubMed]
- 27.Hiraoka K, Fuchiwaki M, Hiraoka K, Horiuchi T, Murakami T, Kinutani M, et al. Re-cryopreservation by vitrification of human blastocysts developed from frozen-cleaved embryos: a report of 15 cycles. J Mamm Ova Res. 2007;24:23–8. [DOI]