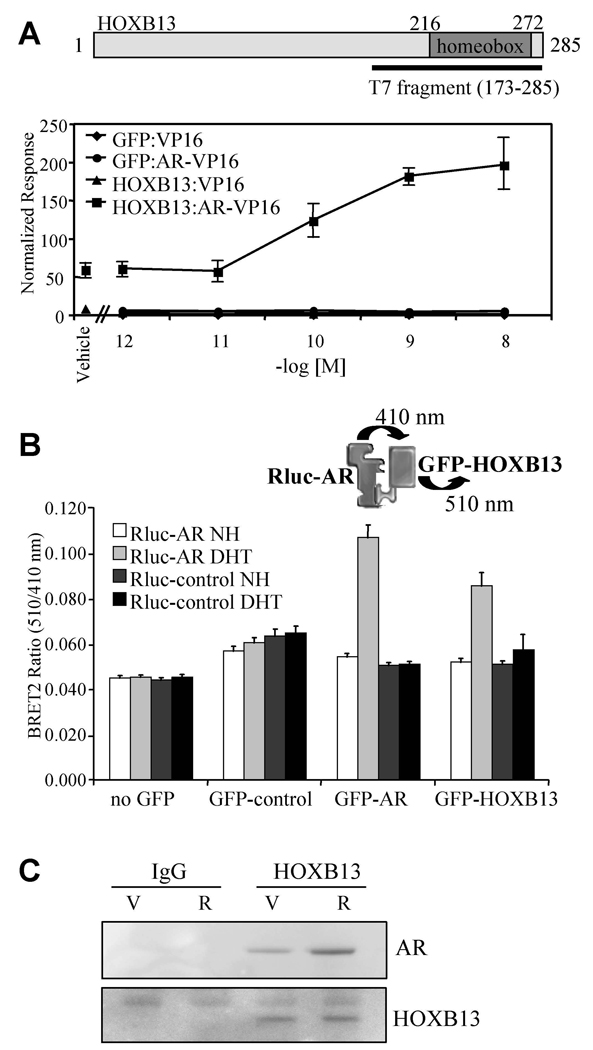
Figure 1. HOXB13 Interacts with AR in Cells.
(A) Mammalian two-hybrid assay. HepG2 cells were transfected with AR-VP16 or VP16 alone, pCMV-β-gal, 5XGalLUC3 (reporter), and either pM-HOXB13 or pM-GFP (Gal4 DNA binding domain fusion protein constructs). Cells were induced for 48 h with R1881 (as indicated) and assayed for luciferase and β-galactosidase activity. Data is presented as normalized response which was obtained by normalizing luciferase activity with β-galactosidase activity. Error presented as +/− SD of triplicate points.
(B) BRET assay. 293T cells were transfected with pRlucC1-AR (luciferase-AR fusion) or pRluc-C1 (luciferase only) together with either no GFP, empty GFP control, GFP-AR or GFP-HOXB13. GFP-AR was used as a positive control in the dimerization assay. Cells were harvested for analysis following 1 h treatment with DHT (100nM) or vehicle control as indicated (NH; vehicle control, DHT; 5α-dihydrotestosterone). Renilla luciferase acts upon the substrate DeepBlue C to emit light at 410 nm which excites the GFP in close proximity to emit light at 510 nm. The interaction between the luciferase and GFP fusion proteins was measured and expressed as BRET ratio (510nm/410nm). Data shown is the average of 6 independent experiments +/− SEM.
(C) Coimmunoprecipitation. LNCaP cells (100mm dishes) were induced with ligand as indicated (V; vehicle, R; 10nM R1881) for 2 h. Immunoprecipitations were performed using either IgG or anti-HOXB13 antibody. The resultant immunocomplexes were resolved using SDS-PAGE and western blot was performed using either anti-AR or anti-HOXB13 antibodies.