Figure 5. G-CSF activation of Erk1/2 requires Jak2 kinase activity.
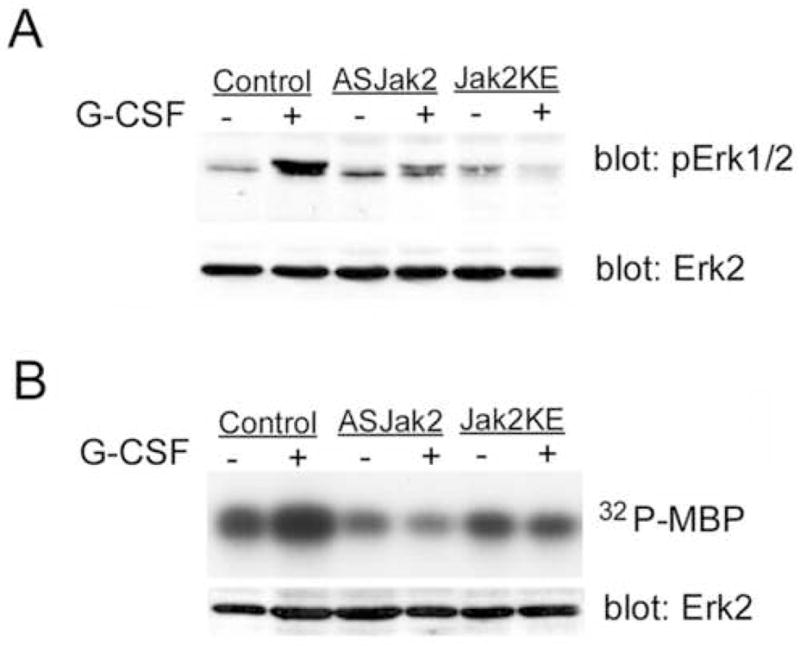
A. DT40GR cells (control) or DT40GR cells stably transfected with either antisense Jak2 (ASJak2) or dominant negative Jak2KE, were treated (+) or not (−) with G-CSF. Lysates were evaluated by SDS-PAGE and Western blotting using antibodies to dually phosphorylated, activated Erk1/2 (pErk1/2) and antibodies to Erk2 protein. The latter (lower panel) demonstrates equivalent amounts of Erk2 in all samples. The upper panel shows the expected phosphorylation of Erk1/2 in response to G-CSF (lane 2) but also reveals (lanes 4 and 6) that G-CSF-dependent phosphorylation was inhibited in cells transduced with Jak2 antisense or the dominant negative Jak2 KE mutant.
B. In vitro kinase activity of Erk from DT40GR cells (control) or from DT40GR cells stably transfected with antisense Jak2 (ASJak2) or dominant negative Jak2 (Jak2KE), treated (+) or not (−) with G-CSF. The Erk substrate myelin basic protein (MBP) and [γ-32P]ATP were added to anti-Erk immunoprecipitates in kinase buffer. After 10 minutes at 30°C, reactions were stopped by boiling in sample buffer. Samples were analyzed by SDS-PAGE and autoradiography to detect phosphorylated MBP (upper panel). Samples from the Erk1/2 immunoprecipitates were also evaluated by Western blotting to confirm equivalent recovery of Erk2 protein in all immunoprecipitates. While Erk from G-CSF-stimulated, wild-type DT40GR cells strongly phosphorylated MBP, G-CSF-stimulation of Erk phosphorylation of MBP was not evident when Erk was immunoprecipitated from cells lacking active Jak2 (ASJak2 and Jak2KE cells).
