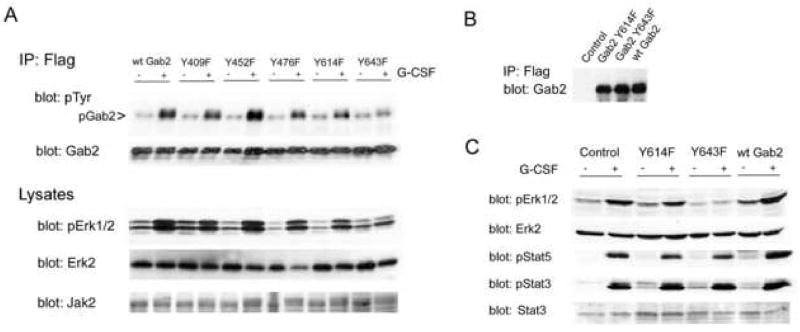
Figure 6. G-CSF-stimulated Jak2-dependent activation of Erk1/2 requires Gab2 tyrosine 643.
A. Human 293 cells were transfected with wild type Gab2 (wtGab2) or a Gab2 tyrosine mutant (identified by the number of the mutated tyrosine: Y409F, Y452F, Y476F, Y614F, Y643F), and with Jak2 and the HA-tagged G-CSF receptor. Cells were stimulated (+) or not (−) with G-CSF for 5 minutes then lysed. Half of each lysate was used for immunoprecipitation of transfected Gab2 with antibodies to its Flag epitope tag. Immunoprecipitates were analyzed by Western blotting for phosphotyrosine and Gab2 (upper panels). G-CSF-stimulated Gab2 phosphorylation appeared to be blocked by mutation of Y643. Samples of each lysate were evaluated by SDS-PAGE and Western blotting with anti-Jak2 (bottom) and sequential Western blotting with anti-Erk2 and anti-phosphoErk1/2 antibodies (middle panels), and antibodies to the HA-tag (not shown), with equivalent expression found in all transfectants. G-CSF-stimulated Erk1/2 phosphorylation was found in cells expressing wild-type Gab2 or any of the Gab2 tyrosine mutants except for the Gab2 Y643F mutant. Thus, mutation of Y643 inhibited not only G-CSF stimulated Gab2 phosphorylation but also G-CSF activation of Erk1/2.
B. DT40GR cells were stably transfected with vector alone (control), the Flag-tagged Y614 Gab2 mutant (Gab2 Y614F), the Flag-tagged Y643 Gab2 mutant (Gab2 Y643F) or the Flag-tagged but otherwise unmutated Gab2 (wt Gab2). For each of these cell lines, the transfected protein was immunoprecipitated from lysates using antibody to the Flag epitope tag. The immunoprecipitates were then analyzed by SDS-PAGE and Western blotting using anti-Gab2 antibody; results demonstrate equivalent expression of wild-type and mutant Gab2 proteins by the transduced cell lines, and an absence of signal from the vector-transfected control cells.
C. The DT40GR cells stably transduced with vector (control), the Gab2 Y614 mutant (Y614F), the Gab2 Y643 mutant (Y643F), or epitope-tagged wild-type Gab2 (wt Gab2) were stimulated (+) or not (−) with G-CSF for 5 minutes then lysed. Cell lysates were analyzed by SDS-PAGE and sequential Western blotting using antibodies to phosphoErk1/2 (pErk1/2) and Erk2 protein (upper panels), phosphoStat5 (pStat5) and phosphoStat3 (pStat3) (lower panels) and Stat3 (data not shown). Mutation of Gab2 Y643 blocked G-CSF-stimulated Erk phosphorylation but had no effect on G-CSF stimulated phosphorylation of Stat3 (pStat3) or Stat5 (pStat5). Equivalent protein loading of all samples is confirmed by the anti-Erk2 immunoblot, which shows equal amounts of Erk2 protein in all samples.