Figure 7. Mutation of Gab2 tyrosine 643 inhibits its in vitro phosphorylation by Jak2.
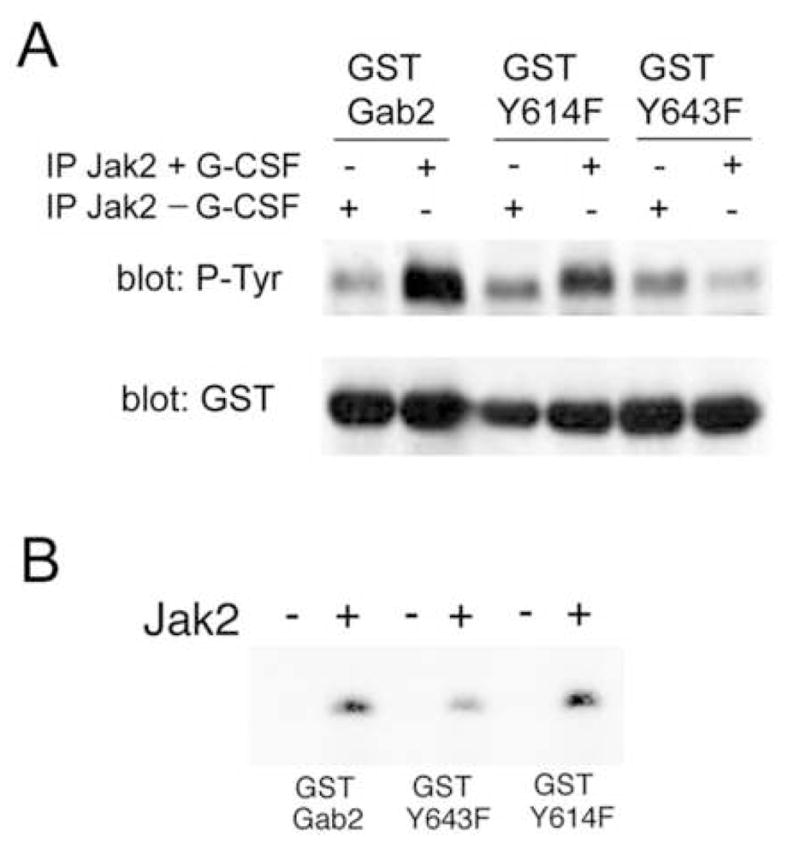
A. Jak2 immunoprecipitated from cells stimulated (+) or not (−) with G-CSF was resuspended in kinase buffer, and aliquots were incubated with GST-Gab2 and GST-Gab2 tyrosine mutants with substitution of phenylalanine at residues corresponding to tyrosines 614 (GST Y614F) and 643 (GST Y643F) in full-length Gab2. After incubation at 30°C for 10 minutes, proteins were analyzed by Western blotting with anti-phosphotyrosine antibodies (upper panel) and anti-GST antibodies (lower panel). Anti-phosphotyrosine revealed strong phosphorylation of the unmutated GST-Gab2 by Jak2 from G-CSF-stimulated cells. Phosphorylation of GST Y614F was also increased over background by Jak2 from stimulated cells, though the increase was less marked. GST Y643F, however, showed no increase in phosphorylation when incubated with G-CSF-stimulated Jak2, supporting the identification of Y643 as a major site of Jak2 phosphorylation. The presence of equal amounts of fusion proteins in each lane was confirmed by the anti-GST immunoblot.
B. GST-Gab2, GST Y643F and GST Y614F were incubated for 30 minutes without (−) or with (+) purified, activated Jak2 in kinase buffer containing radiolabeled ATP. Kinase reactions were then analyzed by SDS-PAGE and autoradiography. These studies revealed that tyrosine phosphorylation by Jak2 was significantly decreased by mutation of Y643, even after prolonged incubation with active Jak2, suggesting that Gab2 Y643 is a major site of direct phosphorylation by Jak2 in vitro.
