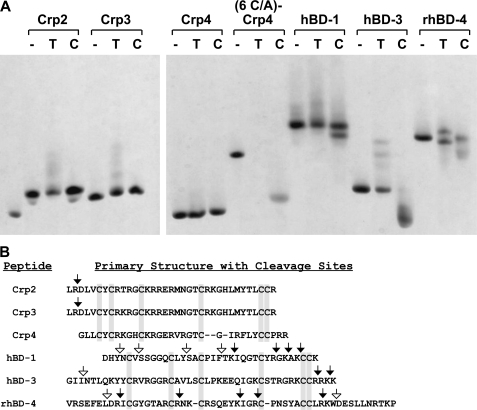
FIGURE 6.
Differential susceptibility of α-defensins and β-defensins to proteolysis by trypsin and chymotrypsin. Representative mouse α-defensins, including Crp2, Crp3, and Crp4, and β-defensins, hBD-1, hBD-3, and rhBD-4, were incubated without enzyme (−), with trypsin (T), or with chymotrypsin (C). (6 C/A)-Crp4, a peptide lacking the disulfide bonds common to all α-defensins, was included as a susceptible control peptide. Samples representing 85% of each digest were analyzed by AU-PAGE (A), and the remainder of each digest was subjected to MALDI-TOF MS to determine the masses of enzymatic cleavage products larger than 2 kDa. In B, the primary structures of the peptides are aligned with the canonical cysteine positions boxed in gray. Trypsin cleavage sites deduced from the MALDI-TOF MS analysis are shown with filled arrows, and chymotrypsin cleavage sites are shown with open arrows.