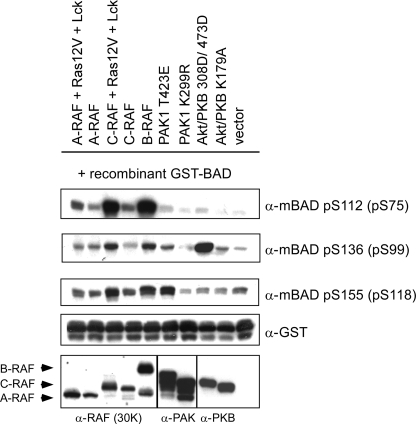
FIGURE 3.
In vitro phosphorylation of recombinant GST-BAD by kinases overexpressed in HEK293 cells. HEK293 cells were transiently transfected with the indicated plasmids. Inactive mutants of PAK1 and Akt/PKB (K299R and K179A, respectively) were used as negative controls. 16 h post-transfection, cells were cultivated for an additional 30 h in medium supplemented with 0.3% serum. Afterward, cells were washed once in PBS and lysed by the direct addition of Nonidet P-40 buffer. For the kinase assay, 35 μg of the protein lysates containing the desired kinases were mixed with 1 μg of recombinant GST-BAD (purified from E. coli) in kinase buffer. Proteins were separated on a 12% SDS-polyacrylamide gel and blotted. Phosphorylation of BAD was visualized with phosphospecific BAD antibodies. Expression levels of RAF kinases, PAK1, and Akt/PKB are shown in the lower panels. This experiment was repeated three times with the same results.