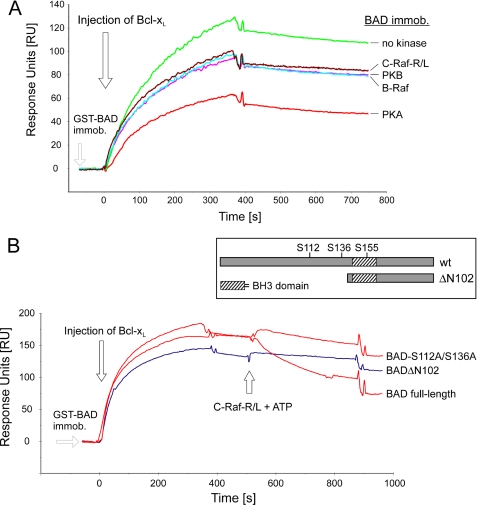
FIGURE 8.
Phosphorylation of recombinant BAD inhibits complex formation between BAD and Bcl-XL and disrupts preexisting complex. A, purified GST-BAD (200 pmol) was phosphorylated by purified and active B- and C-RAF, Akt/PKB, and PKA (20 pmol each) as described under “Experimental Procedures.” Association of phosphorylated BAD with full-length Bcl-XL was monitored using the surface plasmon resonance technique. Approximately 800 response units of GST-BAD phosphorylated with respective kinases were immobilized by anti-GST-coated surface. Bcl-XL (200 nm) was injected, and the association-dissociation curves were monitored. B, approximately 1000 response units of GST-tagged BAD WT, BAD-S75A/S99A (here termed as BAD-S112A/S136A), and BAD(ΔN102) were immobilized, and Bcl-XL (200 nm) was injected. After saturation, the formed complexes were treated with C-RAF-R/L in the presence of ATP. The structure of BAD samples used in this assay is illustrated in the inset.