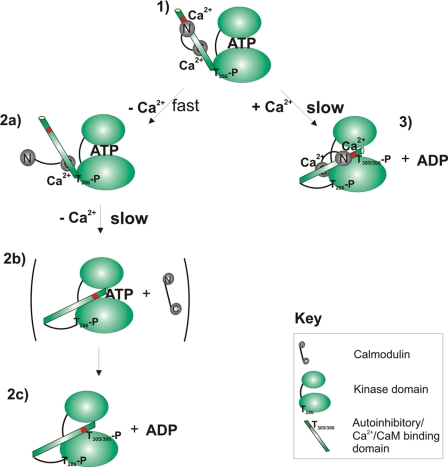
SCHEME 1.
Mechanisms of auto-inactivation of Ca2+/CaM·phospho-Thr286-αCaMKII. Two alternative pathways of autoinactivation leading to Thr305/306 autophosphorylation are illustrated from the perspective of a single kinase domain. The top and bottom ellipses represent the β-sheeted propeller domain and the α-helical substrate binding domain connected by a hinge and with the ATP binding site in between (4). The pathway in the left column includes initial rapid dissociation of the CaM N lobe followed by a slow conformational change accompanying the dissociation of the CaM C lobe, allowing a conformational change permissive for Thr305/306 autophosphorylation to occur. This path results in an inactivated enzyme complex free of CaM. 1, active Ca2+/CaM·phospho-Thr286-αCaMKII; the autoinhibitory domain is detached from the kinase domain and is Ca2+/CaM-bound. 2, upon Ca2+ chelation by EGTA, rapid dissociation of the N lobe of CaM is seen. 2b, slow dissociation of CaM C lobe; slow conformational change positions Thr305/306 as a “canonical” substrate, adjacent to the ATP binding pocket. 2c, Thr305/306 autophosphorylation of Ca2+/CaM-free kinase. In the right-hand column, Thr305/306 autophosphorylation of the Ca2+/CaM-bound phospho-Thr286-αCaMKII is shown. 3, slow conformational change leads to the conformation permissible for Thr305/306-autophosphorylation.