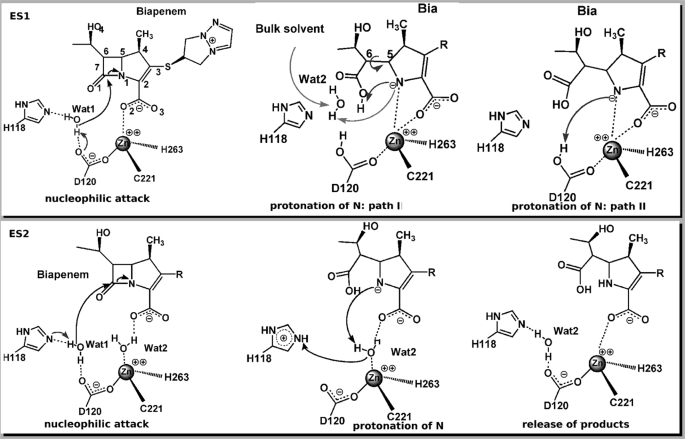
FIGURE 1.
Schematic representation of the proposed reaction mechanisms of B2 MβL CphA. Top panel, the Henry-Michaelis complex ES1 assumes direct binding of the substrate to the zinc ion and one water molecule (Wat1) in the active site. Wat1 is deprotonated by a general base (His118 or Asp120) to perform the nucleophilic attack (15). Nitrogen protonation can take place by a second water molecule (Wat2), involving also an internal rearrangement in the substrate (Path I) or from the general base (in this case, Asp120 is shown as the general base and proton donor) (Path II). Bottom panel, in the Henry-Michaelis complex ES2, the interaction of the substrate with the zinc ion is mediated by a water molecule (Wat2), whereas Wat1 occupies a similar position as in ES1. Wat1 is activated by a general base (His118 in this picture) to perform the nucleophilic attack. The zinc-bound Wat2 acts as a proton donor to the lactam nitrogen.