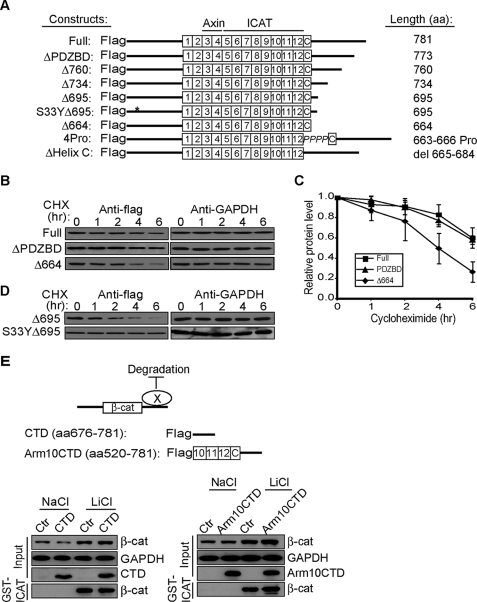
FIGURE 1.
The flexible CTD is required for β-catenin stability. A, schematic representation of FLAG-tagged β-catenin mutants. B, turnover rates of β-catenin mutants. HEK293T cells were transfected with 1.0 μg of plasmids 48 h before chasing in 20 μg/ml cycloheximide (CHX) for the times indicated. Total cell lysates were immunoblotted for FLAG tag and endogenous GAPDH. C, quantification of triplicate experiments as shown in panel B. β-Catenin levels were normalized to GAPDH from the same blot. The ratio at time 0 was set to 1.0. D, stabilization of FLAG-β-catenin Δ695 by mutating serine 33 to tyrosine. E, overexpression of β-catenin CTD does not affect stability of endogenous β-catenin. Upper panel, schematic representation of model, where CTD binds protein X to antagonize degradation or promote stability. Unstructured CTD (amino acid (aa) 676–781) and CTD that includes arm repeats 10–12 and Helix C (arm10CTD) are shown. Lower panels, HEK293T cells were transiently transfected with CTD constructs and 48 h later treated with 30 mm NaCl or LiCl overnight. Changes in the cytosolic signaling pool of β-catenin were followed by affinity precipitation with GST-ICAT (31, 38). GAPDH levels show equivalent protein loading.