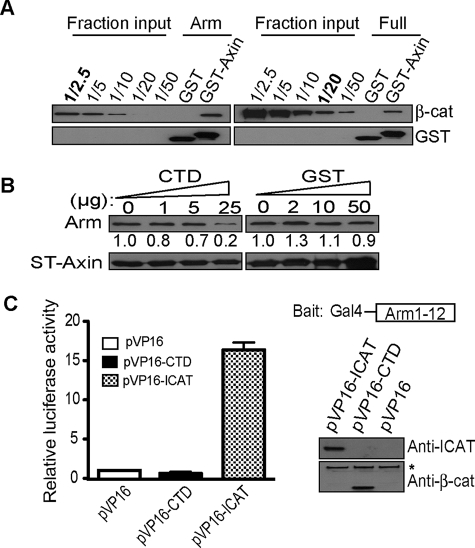
FIGURE 2.
The flexible CTD restricts β-catenin from binding to axin in vitro. A, β-catenin armadillo region alone (His-arm) exhibits better binding to axin compared with full-length β-catenin. 2.0 μg of GST-axin-CBD; amino acids 436–498) pre-coupled glutathione beads were incubated with 0.7 μg of His-arm or 1.0 μg of full-length β-catenin (12 pmol each) purified from baculovirus. Affinity precipitates were separated on SDS-PAGE and loaded in parallel with fractional inputs and immunoblotted for β-catenin to evaluate binding. Note that ∼4.8 pmol of His-arm is pulled down by GST-axin-CBD in comparison with 0.6 pmol of full-length β-catenin. B, CTD, but not GST, competes β-catenin binding to GST-axin-CBD. 2.0 μg of GST-axin-CBD coupled-Sepharose beads were incubated with 1.0 μg of His-β59 in the presence of increasing amounts of β-catenin CTD peptide (amino acids 695–781) or molar equivalents of GST. CTD competes arm-repeat binding to axin at 150-fold molar excess. Affinity precipitates were separated on SDS-PAGE and immunoblotted for the histidine tag. Band intensities calculated with Image J program are shown under corresponding gel bands. The number obtained without competitor peptide was set to 1.0. C, β-catenin CTD does not interact with arm-repeat region of β-catenin in trans. Mammalian two-hybrid assay of pM-arm (amino acid 140–663) and pVP16-CTD. Right, schematic of the Gal4-arm-(1–12) “bait” and immunoblot verifying hybrid proteins. The asterisk (*) denotes endogenous β-catenin.