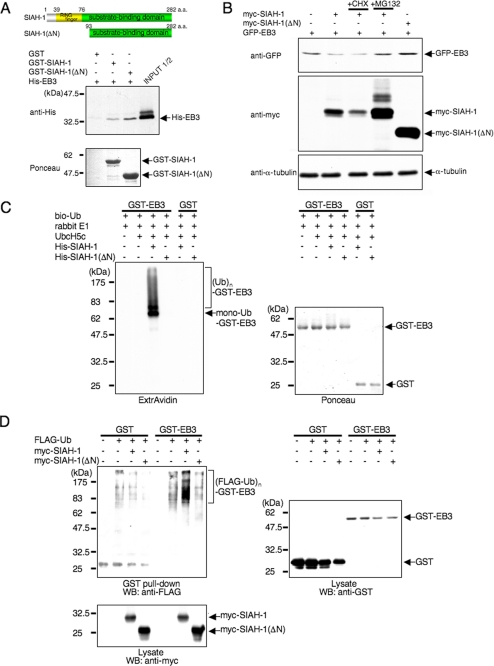
FIGURE 4.
SIAH-1 is E3 for EB3. A, schematic representation of full-length SIAH-1 and SIAH-1(ΔN) (upper panels) and in vitro binding of EB3 and SIAH-1 (lower panels). Purified His-EB3 was incubated with GST-fused, full-length SIAH-1 and SIAH-1(ΔN) trapped on glutathione-Sepharose beads. The bound EB3 was then eluted and detected by Western blots with anti-penta-His antibody (middle panel). The bottom panel shows Ponceau staining of the GST fusion protein samples used in the assay. a.a., amino acids. B, SIAH-1 targets EB3 for proteasomal degradation. COS-7 cells were transfected with the indicated plasmids. After 48 h, cells were treated with or without 10 μg/ml CHX or 20 μm MG132 for 6 h. Using these cell lysates, Western blots were performed with the indicated antibodies. C, SIAH-1-mediated ubiquitination (Ub) of EB3 in vitro (left panel). The ubiquitination reaction was performed as described under “Experimental Procedures.” The ubiquitinated proteins in the reaction were detected by Western blots using ExtrAvidin. The right panel shows Ponceau staining of the GST fusion protein samples used in the assay. D, SIAH-1-mediated EB3 ubiquitination in cells. COS-7 cells were transfected with the indicated plasmids. After 48 h, cells were treated with 20 μm MG132 for 6 h. GST pulldown was performed, and the precipitates were analyzed by Western blots (WB) probed with anti-FLAG antibody. The results shown are from one representative experiment out of at least three independent experiments.