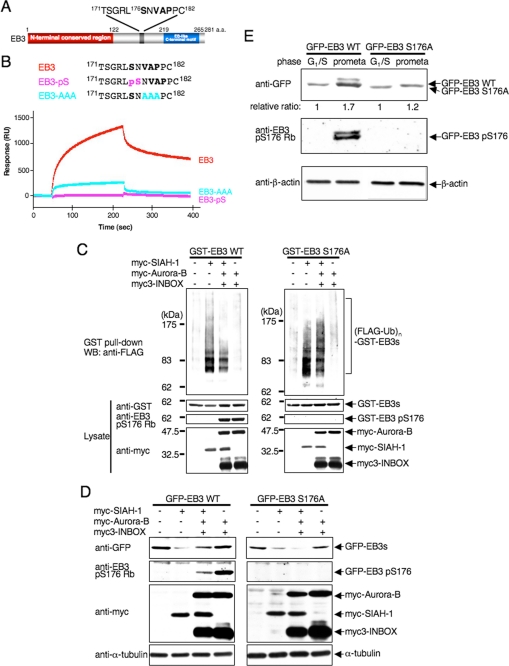
FIGURE 6.
Auroras regulate SIAH-1-dependent EB3 degradation through Ser-176 phosphorylation of EB3. A, schematic diagram of a potential SIAH-binding motif (V178AP180) in EB3. Ser-176 is an Aurora phosphorylation site. a.a., amino acids. B, top, schematic diagram of the sequences of wild-type and mutant peptides derived from EB3. Bottom, a BIAcore sensorgram shows that wild-type EB3 peptides, but not mutant EB3 peptides, bind to immobilized SIAH-1. A representative result of four independent experiments is shown. C, effects of Aurora phosphorylation on SIAH-1-mediated ubiquitination of EB3 in cells. COS-7 cells were transfected with each plasmid (as indicated above the figure). The ubiquitinated proteins were detected as described in Fig. 4D. WT, wild type. D, effects of Aurora phosphorylation on SIAH-1-mediated EB3 degradation. COS-7 cells were transfected with each plasmid (as indicated above the figure). After 48 h, cells were harvested, and Western blots (WB) were performed with the indicated antibodies. E, effects of Aurora phosphorylation on the stability of the EB3 proteins in mitosis. HeLa cells that stably expressed each GFP-wild-type EB3 or EB3 S176A were synchronized at each phase. Subsequently, cells were treated with CHX for 6 h, and the cell lysates were subjected to Western blots with the indicated antibodies. The relative protein level of GFP-EB3 at G1/S phase against the prometaphase is shown below each lane. C–E are representative of three independent experiments.