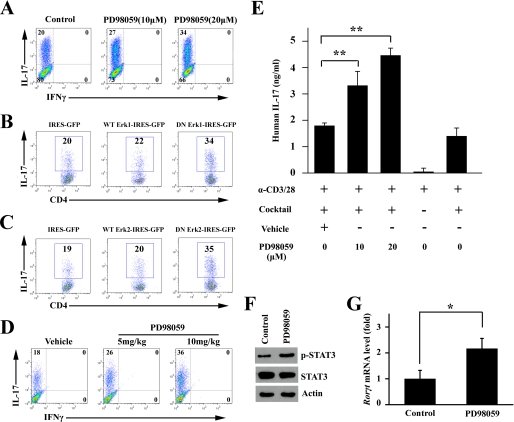
FIGURE 5.
ERK negatively regulated in vitro Th17 differentiation. A, naive CD4+ T cells from NOD mice were cultured under the Th17 condition in the absence or presence of PD98059 at the indicated concentration for 4 days. Intracellular staining profiles for IL-17 and IFNγ are shown. B and C, retroviral expression of the DN form of ERK1 (B) and ERK2 (C) promoted Th17 differentiation. The WT and DN forms of ERK1 and ERK2 were cloned into the retroviral vector pMXs-IRES-GFP. Plasmids were transfected into Platinum-E to generate a retrovirus supernatant. Naive CD4+ T cells were activated with TCR stimulants and the Th17 mixture for 2 days before infection with the retrovirus-containing Platinum-E supernatant and then cultured for a further 2 days. The gate was set on GFP+ CD4+ T cells to study IL-17 production. D, 11-week-old female NOD mice were intraperitoneally injected with vehicle or PD98059 (5 or 10 mg/kg) for 3 consecutive days (one time each day) and were then sacrificed. Naive CD4+ T cells were isolated and cultured under the Th17 condition for 4 days. Intracellular staining profiles of IL-17 and IFNγ are shown. E, human CD4+ T cells were isolated from healthy donors and cultured under the Th17 condition in the presence of PD98059 at the indicated concentration for 3 days. The supernatant was collected for ELISA tests. The concentration of IL-17 was normalized for cell number (**, p < 0.01, n = 3). F and G, naive CD4+ T cells from NOD mice were cultured under the Th17 condition in the absence or presence of PD98059 for 4 days. Cell lysates were subjected to Western blotting (F) and real time PCR after RNA extraction and reverse transcription to cDNA (G). The gene expression is presented relative to that of β-actin (*, p < 0.05).