FIGURE 2.
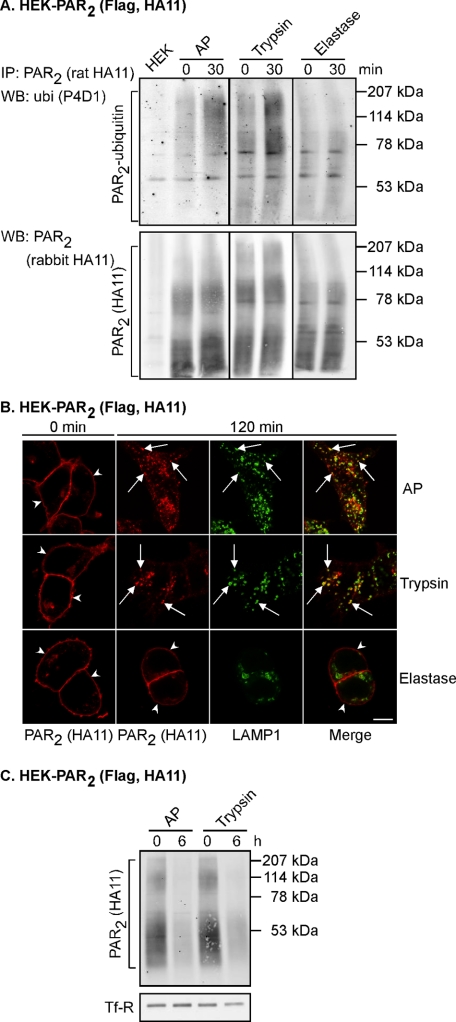
AP and trypsin, but not elastase, induce PAR2 ubiquitination, trafficking to lysosomes, and degradation. A, HEK-PAR2 cells were stimulated for 0 or 30 min with AP (100 μm), trypsin (10 nm), or elastase (0.5 μm). PAR2 was immunoprecipitated (IP), and membranes were analyzed by Western blotting (WB) for ubiquitin (ubi) and PAR2. Only AP and trypsin induced ubiquitination of PAR2. B, HEK-PAR2 cells were challenged for 0 or 120 min with AP (100 μm), trypsin (10 nm), or elastase (0.5 μm). Cells were fixed, and proteins were localized by indirect immunofluorescence with antibodies to HA11 (PAR2) and LAMP1. In unstimulated cells, PAR2 was located at the plasma membrane (arrowheads) and in a perinuclear pool. Stimulation with AP or trypsin induced PAR2 translocation to LAMP1-containing vesicles (arrows). In contrast, PAR2 remained at the plasma membrane following treatment with elastase (arrowheads). Scale bar, 10 μm. C, cell surface proteins on HEK-PAR2 cells were biotinylated prior to stimulation with AP (100 μm) or trypsin (10 nm) for 0 or 6 h. Biotinylated proteins were precipitated using avidin beads, and PAR2 and TfR were detected by Western blotting. Stimulation with AP or trypsin both induced degradation of PAR2. Results shown representative of n = 3 experiments.