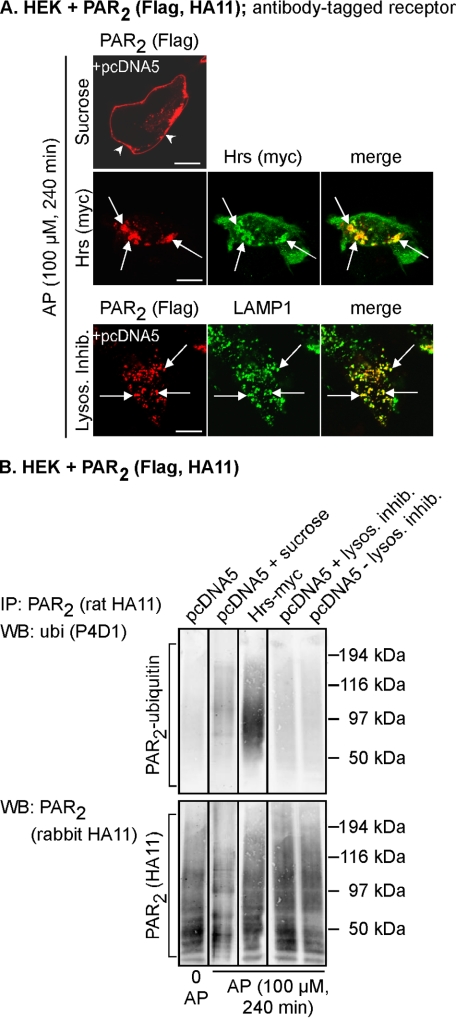
FIGURE 3.
PAR2 is ubiquitinated at the plasma membrane and early endosomes and deubiquitinated before transfer to lysosomes. HEK cells were transiently transfected with PAR2 and pcDNA5 (control) or Hrs-Myc. A, cell surface PAR2 was labeled with antibody to extracellular FLAG epitope. Cells were stimulated with AP (100 μm, 240 min), and PAR2, Hrs, and LAMP1 were localized. In cells treated with hypertonic sucrose, PAR2 remained at the plasma membrane (arrowheads). Expression of Hrs prevented trafficking of PAR2 from early endosomes. In cells treated with lysosomal protease inhibitors, PAR2 accumulated in LAMP1-positive lysosomes (arrows). Scale bar, 10 μm. B, cells were treated as in A. PAR2 was immunoprecipitated (IP), and membranes analyzed by Western blotting (WB) for ubiquitin (ubi) and PAR2. In unstimulated cells, there was no detectable PAR2 ubiquitination. AP-induced PAR2 ubiquitination was detected when trafficking of PAR2 was blocked at the plasma membrane with sucrose or when PAR2 was trapped in early endosomes by overexpression of Hrs. PAR2 ubiquitination was not detected when PAR2 had accumulated in lysosomes in the presence or absence of lysosomal inhibitors. Results shown are representative of n = 3 experiments.