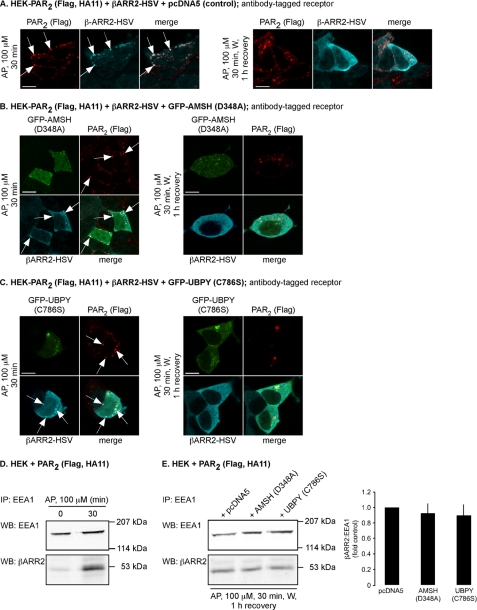
FIGURE 9.
Expression of catalytically inactive AMSH or UBPY does not prolong the interaction between β-arrestin2 and PAR2 in endosomes. HEK-PAR2 cells were transiently transfected with β-arrestin2-HSV and pcDNA5 (control) (A), GFP-AMSH(D348A) (B), or GFP-UBPY(C786S) (C). Cell surface PAR2 was labeled with FLAG antibody. Cells were challenged with AP (100 μm, 30 min) or with AP (100 μm, 30 min), followed by washing and recovery in AP-free medium for 1 h. In control cells, AP (30 min) induced trafficking of PAR2 and β-arrestin2 to the same vesicles (presumably endosomes; arrows). After a 1-h recovery, PAR2 was still present in vesicles, and β-arrestin2 had returned to the cytosol. Similar results were obtained in cells expressing GFP-AMSH(D348A) or GFP-UBPY(C786S). Scale bars, 10 μm. D, HEK cells transiently transfected with PAR2 were stimulated with AP (100 μm) for 0 or 30 min. Endosomes were isolated and analyzed for β-arrestin2 and EEA1 by Western blotting. AP (30 min) increased the levels of β-arrestin2 in endosomes. E, HEK cells transiently transfected with PAR2 and pcDNA5 (control), GFP-AMSH(D348A), or GFP-UBPY(C786S) were challenged with AP (100 μm, 30 min), followed by washing and recovery in AP-free medium for 1 h. Endosomes were isolated and analyzed for β-arrestin2 and EEA1 by Western blotting and quantified using densitometry. Expression of GFP-AMSH(D348A) or GFP-UBPY(C786S) did not cause retention of β-arrestin2 in endosomes. n = 3. IP, immunoprecipitation; WB, Western blot.