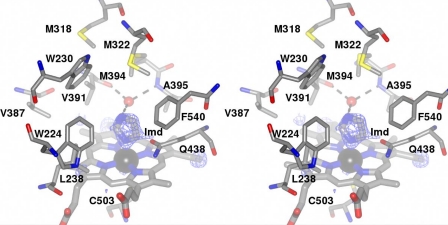
FIGURE 4.
Stereoview of the environment surrounding the heme in XplA-heme. The ceiling of the heme binding site is notably hydrophobic and features an unusual cluster of methionine residues, Met-318, Met-322, and Met-394, the latter held over the heme with the tip of the side chain at a distance of 8.2 Å from the iron. Ligand electron density observed at the distal face of the heme revealed the presence of imidazole. The density corresponds to the omit map (Fo − Fc map contoured at a level of 3.2ς) obtained after structure solution using the native enzyme as a search model in MOLREP (17). H-bonding interactions between the imidazole ligand and the water molecule (shown as a red ball) that occupies the cavity between the peptidic N–H of Ala-395 and the peptidic carbonyl group of Val-391 are shown as dashed black lines. The side chain of Gln-438, hanging over the heme and at a distance of ∼3.5 Å from the imidazole ligand, can also be seen.