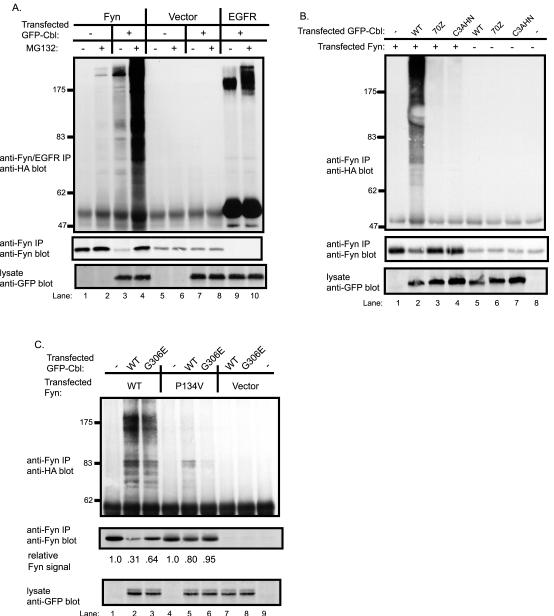
Fig. 3. Cbl-dependent ubiquitination of Fyn in 293T cells and an essential role for the Cbl RING finger domain.
A, Cbl-dependent Fyn ubiquitination is enhanced by treatment with a proteasome inhibitor. 293T cells were transfected with plasmids encoding HA-ubiquitin (7 μg), Fyn (0.15 μg), EGFR (0.15 μg), GFP-Cbl (+) (3 μg) or a GFP (−) control (3 μg). Five hr prior to cell lysate preparation, cells were treated with 50μM MG132 (+) or DMSO control (−). Anti-Fyn or anti-EGFR immunoprecipitates from aliquots of lysate protein (800 μg) were immunoblotted with anti-HA antibody (top panel) followed by anti-Fyn antibody (middle panel). Equal aliquots (30 μg) of the same cell lysates used above were immunoblotted with anti-GFP antibody (bottom panel). B, an intact RING finger domain is required for Cbl-dependent Fyn ubiquitination. 293T cells were transfected with the indicated expression plasmids, lysed and anti-Fyn immunoprecipitations were carried out as in A and immunoblotted with anti-HA antibody (top panel) and with anti-Fyn antibody (middle panel). The lysate proteins (30 μg) were immunoblotted with anti-GFP antibody (bottom panel). C, the role of Fyn SH3 domain and Cbl TKB domain-mediated interaction for Cbl-dependent Fyn ubiquitination. 293T cells were transfected with the vector, wildtype Fyn or Fyn-SH3 domain mutant (P134V), lysed and anti-Fyn immunoprecipitations were carried out as in A and immunoblotted with anti-HA antibody (top panel) and with anti-Fyn antibody (middle panel). The lysate proteins (10 μg) were immunoblotted with anti-GFP antibody (bottom panel). The levels of Fyn protein were quantified by densitometry, and the values are expressed as a function of the initial Fyn protein level for each Fyn construct (lane 1 and 4) that was assigned a value of 1.0.