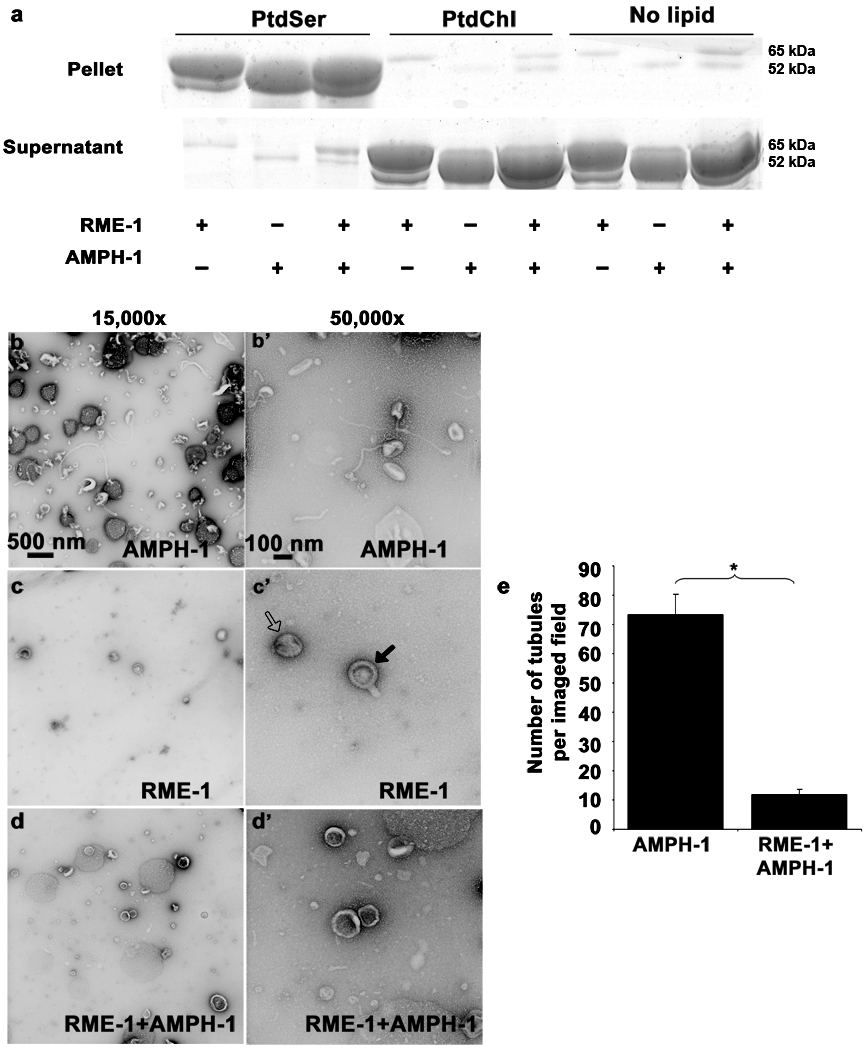
Figure 8.
Nucleotide effects on RME-1 and AMPH-1 mediated liposome tubulation. (a) Coomassie stained gels of supernatant and pellet fractions from liposome co-sedimentation assays are shown. Binding reactions were performed in the absence or presence of 0.33 mg/ml, 0.4 µm (average diameter) 100% Phosphatidylserine (PtdSer), or 100% Phosphatidylcholine (PtdChl) liposomes. Liposomes were incubated with 1mM ADP and 1 µM of full length AMPH-1 or RME-1 proteins, or equimolar quantities of both proteins, as indicated. Note that RME-1 can bind to PtdSer liposomes in the presence of ADP. Tubulation experiments were performed with 2.5µM of each protein and 1mM ADP incubated with 0.05mg/ml 100% PtdSer liposomes. (b-b’) AMPH-1 incubated with PtdSer liposomes in the presence of ADP (compare with ATP-γ-S, Fig. 5 c-c’). (c-c’) RME-1 incubated with PtdSer liposomes in the presence of ADP (compare with ATP-γ-S, Fig. 5 d-d’). RME-1(ADP) lacks tubulation capacity and most liposomes remain spherical (open arrow) in the presence of RME-1(ADP). Striations are often visible and rare short protrusions (closed arrows) are present on occasional liposomes. (d-d’) RME-1 in its ADP bound state can affect the tubulation ability of AMPH-1 as observed in an experiment containing equimolar concentrations of both proteins in the presence of ADP. (e) Quantification reveals an approximately 7 fold decrease in number of tubules in conditions where RME-1 is present with AMPH-1, as compared to tubulation produced by AMPH-1 alone. n=10 fields for each experimental condition (imaged at 3,000× magnification), the mean value for number of observed tubules was plotted and error bars represent ± s.d. from the mean. The asterisk indicates a significant difference in the one-tailed Student’s T-test (p value=1.48×10−15).