Table 1.
Equilibrium Constants in D2O for Addition of Glycine to the Aromatic Aldehydes 1–4.a
| aldehyde | iminium ion | p(Ka)IMb | p(Ka)ODc | (Kadd) d (M−1) | (Kadd)+e (M−1) | (Kadd)++f (M−1) |
|---|---|---|---|---|---|---|
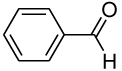 |
 |
6.29 ± 0.01 | 44 ± 8 | (3.3 ± 0.4) × 10−3 | ||
 |
 |
12.13 ± 0.05 | 5.55 ± 0.04 | 1.76 ± 0.02 | 72 ± 8 | (3.4 ± 0.1) × 10−2 |
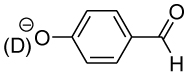 |
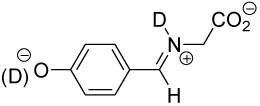 |
10.16 ± 0.03 | 6.61 ± 0.02 | 0.49 ± 0.03 | 0.29 ± 0.03 | (8.7 ± 0.9) × 10−3 |
 |
 |
4.95 ± 0.06 | (500 ± 20) | (2.0 ± 0.4) × 10−3 |
At 25 °C and I = 1.0 (KCl).
Apparent pKa of the iminium ion nitrogen in D2O, determined by NMR titration as described in the text.
Apparent pKa of the iminium ion phenol group in D2O, determined by NMR titration as described in the text.
Equilibrium constant for addition of the basic amino form of the amino acid to the basic form of the aldehyde.
Equilibrium constant for addition of the amino acid zwitterion to the basic form of the aldehyde.
Equilibrium constant for addition of the amino acid zwitterion to the protonated form of the aldehyde.
