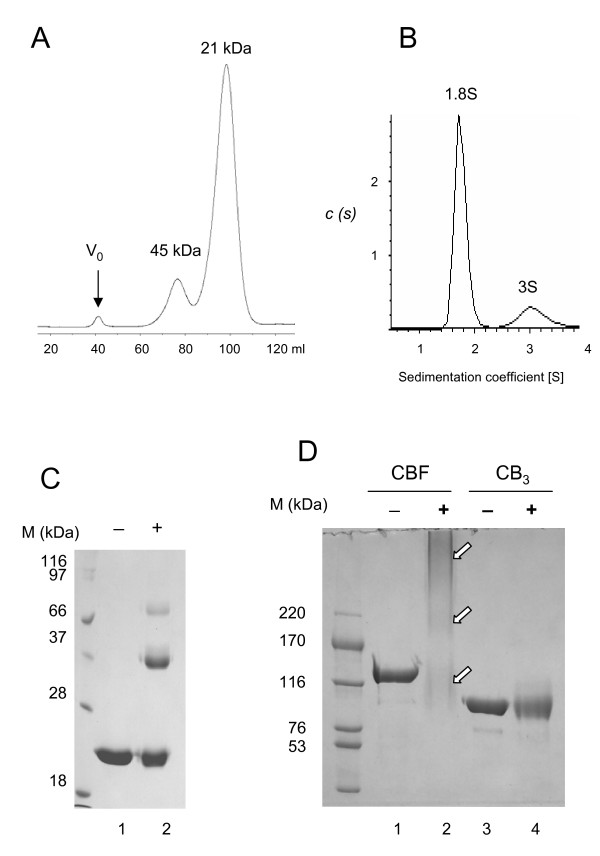
Figure 1.
Monomer to dimer ratio of the F5/8C module of LamA. (A) Elution profile of the purified protein using a Sephacryl S-300 column. The Vo indicates the void volume. The estimated molecular masses of the eluted peaks are indicated. (B) Sedimentation velocity studies. The condition for analytical ultracentrifugation and data analysis are described in the Materials and Methods. (C) Glutaraldehyde cross-linking of the purified F5/8C module. The protein (0.65 mg/mL) was treated with 0.05% glutaraldehyde at room temperature for 1 h and resolved on a 12% SDS-PAGE gel. Lanes 1 and 2 contain the protein sample without and with the treatment of glutaraldehyde, respectively. (D) Glutaraldehyde cross-linking of two larger truncated proteins of LamA (CBF and CB3). The proteins (0.3 mg/mL) were treated with 0.05% glutaraldehyde at room temperature for 40 min and resolved on an 8% SDS-PAGE gel. The arrows point to the putative migration zones of monomeric, dimeric, and multimeric CBF after glutaraldehyde treatment.