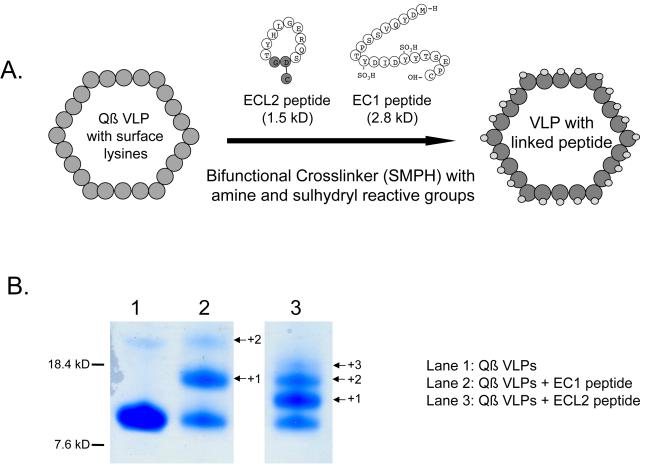
Figure 1.
Generating the CCR5 vaccines. A) EC1 and ECL2 peptides were linked to Qß VLPs through the use of a bifunctional crosslinker (SMPH). SMPH crosslinks surface lysines on Qß VLPs to a cysteine located at the C-terminus of the EC1 peptide or the base of the cyclized ECL2 peptide. Non-CCR5 derived amino acids are highlighted in grey. Numerous copies of peptide can be attached per coat protein, resulting in peptide presentation in a dense and repetitive array on the VLP surface. B) Polyacrylamide gel analysis of denatured Qß VLPs (lane 1), EC1-conjugated Qß VLPs (lane 2), and ECL2-conjugated Qß VLPs (lane 3). Qß VLPs are comprised of a single protein subunit, coat protein, which migrates with a mobility corresponding to its molecular weight, ~14000 Daltons. Conjugation of the EC1 peptide results in higher molecular weight species, representing individual coat protein subunits modified with 1 (+1), 2 (+2), or 3 (+3) copies of the peptide.